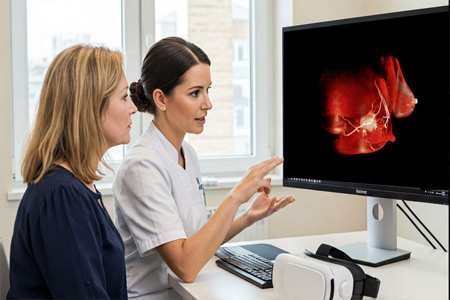
Avatar Medical Vision Receives CE Marking
Avatar Medical, a leader in immersive medical imaging, announces that it has obtained CE marking for its innovative solution, Avatar Medical Vision*. This certification marks a decisive step in the company's mission to democratize access to medical images for both surgeons and patients. Avatar Medical Vision enables the real-time conversion of patient medical images into stunning 3D and XR renderings, enhancing visualization and understanding.
- Details
- Written by: Avatar Medical
- Company Name: Avatar Medical
- Company/Org Logo:

- About Company: Founded in 2020 as a spin-off from the Institut Pasteur and Institut Curie, Avatar Medical democratizes access to medical imaging through advanced 3D visualization software. With offices in Mountain View (California, USA), Paris and Saint-Malo (France), Avatar Medical redefines patient-doctor consultations by enhancing understanding, reducing anxiety, and improving decision-making.
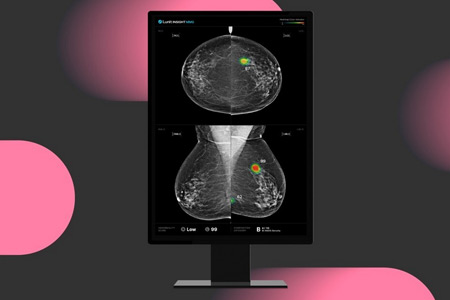
The world’s first large-scale, multicenter prospective study in single-reading mammography confirms that Lunit AI boosts cancer detection
Lunit a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announced the publication of a groundbreaking prospective study in Nature Communications validating the real-world effectiveness of AI-powered mammography screening in South Korea's national breast cancer screening program.
- Details
- Written by: Lunit
- Company Name: Lunit
- Company/Org Logo:

- About Company: Founded in 2013, Lunit (KRX:328130.KQ) is a medical AI company on a mission to conquer cancer. Lunit harnesses AI-powered medical image analytics and biomarker analysis to ensure accurate diagnosis and optimal treatment for each cancer patient. The FDA-cleared Lunit INSIGHT suite for cancer screening serves over 4,800 medical institutions across 55+ countries. Lunit clinical studies have been published in top journals, including the Journal of Clinical Oncology and the Lancet Digital Health, and presented at global conferences such as the ASCO and RSNA. Headquartered in Seoul, South Korea, with a network of offices worldwide, Lunit leads the global fight against cancer.

Silicon Labs unveils BG29, the future of Bluetooth® LE in miniature devices
Silicon Labs the leading innovator in low-power wireless, announced its new Series 2 BG29 family of wireless SoCs, designed to bring high compute and connectivity to the smallest form factor Bluetooth Low Energy (LE) devices without compromising performance. BG29 is ideal for today's most intensive Bluetooth LE applications like wearable health and medical devices, asset trackers, and battery-powered sensors.
- Details
- Written by: Silicon Labs
- Company Name: Silicon Labs
- Company/Org Logo:

- About Company: Silicon Labs (NASDAQ: SLAB) is the leading innovator in low-power wireless connectivity, building embedded technology that connects devices and improves lives. Merging cutting-edge technology into the world's most highly integrated SoCs, Silicon Labs provides device makers with the solutions, support, and ecosystems needed to create advanced edge connectivity applications. Headquartered in Austin, Texas, Silicon Labs has operations in over 16 countries and is the trusted partner for innovative solutions in the smart home, industrial IoT, and smart cities markets.

Qualia supplements achieve multiple finalist honors for product excellence
Since 2010, the NEXTY Awards have honored the highest quality natural products in the world. This year, Qualia Life Sciences is the only supplement brand to have two products, Qualia NAD+ and Qualia Mind.
- Details
- Written by: Qualia Life Sciences
- Company Name: Qualia Life Sciences
- Company/Org Logo:

- About Company: Qualia Life Sciences was established in 2015. Their products emphasize complex systems science, which supports the body's ability to self-regulate and heal - as a key factor in addressing various health issues. Their product lineup features capsule supplements, drink shots, and powdered drink mixes to support health needs such as aging and longevity, brain health, energy, and sleep, with many additional products in development. *Disclaimer: These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. These statements are not intended as general medical advice. This product is not a replacement for prescription medication. Please consult your physician before taking any dietary supplements.
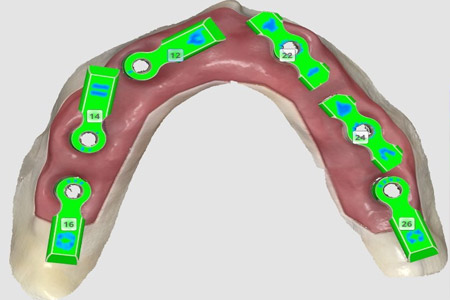
Medit's game-changing workflow for Revolutionizing All-on-X will launch soon
Medit a global leader in digital dentistry, is set to revolutionize the digital All-on-X workflow, making it more accessible, efficient, and intuitive for Medit scanner users.
- Details
- Written by: Medit
- Company Name: Medit
- Company/Org Logo:

- About Company: MEDIT is a global provider of 3D intraoral scanners and an all-in-one digital dentistry platform, based on its own patented state-of-the-art technology. The company also develops innovative software for digital dentistry, supporting collaborative workflows between dental clinics and labs. MEDIT has been headquartered in Seoul, South Korea since its inception in 2000. The company also has representatives located in the Americas and Europe and boasts a global network of distributors in over 100 countries.

Respiree secures Singapore HSA clearance for its pediatric expansion of the cardio-respiratory wearable and its new EHR streamlined platform
AI/ML health tech startup Respiree has received clearance from the Health Sciences Authority (HSA) to extend its RS001 cardio-respiratory wearable to pediatric populations. This clearance also enables the extension of Respiree's software platform, introducing enterprise-grade pathway management services and EHR interoperability which further enhances its innovative healthcare solutions.
- Details
- Written by: Respiree
- Company Name: Respiree
- Company/Org Logo:

- About Company: Respiree™ is an AI/ML health tech company building clinically-validated AI for managing disease progression. Respiree™ is CE marked and has received regulatory clearances from the Therapeutic Goods Administration and the United States Food and Drug Administration (FDA).
