Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: W. L. Gore & Associates, Inc
- Category: Press Releases
W. L. Gore & Associates (Gore) has announced that the first U.S. patient has been enrolled in a prospective, non-randomized, multicenter, single-arm study with five-year follow-up (NCT05489588) to evaluate the investigational GORE® VIAFORT Vascular Stent for the treatment of symptomatic iliofemoral venous obstruction.
- Company Name: W. L. Gore & Associates, Inc
- About Company: W. L. Gore & Associates is a global materials science company dedicated to transforming industries and improving lives. Since 1958, Gore has solved complex technical challenges in demanding environments — from outer space to the world's highest peaks to the inner workings of the human body. With more than 12,000 Associates and a strong, team-oriented culture, Gore generates annual revenues of $4.5 billion.
- Details
- Written by: Esaote North America
- Category: Press Releases
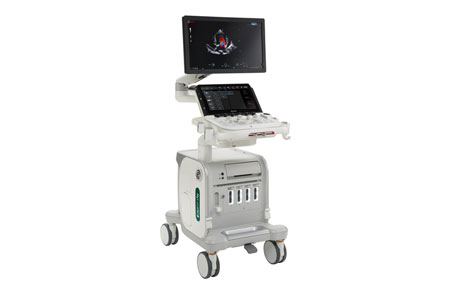
Esaote North America, Inc. is proud to announce the release of their new veterinary MyLab™X90VET ultrasound system, now available for purchase in North America.
- Company Name: Esaote North America
- Company/Org Logo:

- About Company: Esaote North America, as part of the international Esaote Group, continues to develop and distribute innovative medical imaging systems with the support of one of the world's leading medical imaging companies. Esaote S.p.A. is a leader in medical device manufacturing in Ultrasound, Dedicated MRI, and Healthcare IT. Esaote's headquarters are in Genoa, Italy, with an international presence in 100 countries.
- Details
- Written by: SHL Medical
- Category: Press Releases
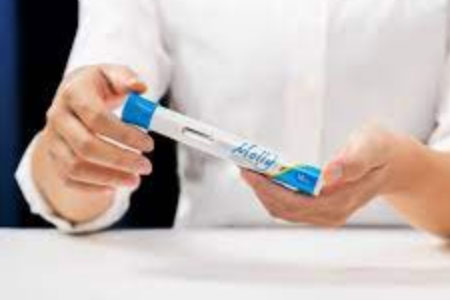
SHL Medical, a world-leading provider of advanced drug delivery solutions, announced that it has signed a collaboration agreement with MoonLake Immunotherapeutics, a clinical-stage biotechnology company focused on creating next-level therapies for inflammatory diseases, to develop an autoinjector for clinical and potential commercial supply of MoonLake's Nanobody® sonelokimab based on SHL Medical's Molly® autoinjector technology.
- Company Name: SHL Medical
- Company/Org Logo:

- About Company: SHL Medical is a world-leading solutions provider in the design, development, and manufacturing of advanced delivery devices such as autoinjectors, pen injectors, and innovative specialty delivery systems for large-volume and high-viscosity formulations. It offers final assembly, labeling, and packaging solutions for its drug delivery devices, and also provides contract manufacturing and engineering services for the production of complex medtech and industrial products. With locations in Switzerland, Taiwan, Sweden, and the US, SHL Medical's experienced designers and engineers develop product enhancements and breakthrough drug delivery solutions for pharma and biotech clients globally. Significant investments in R&D have enhanced its broad pipeline of next-generation drug delivery systems that support ongoing innovations in drug development and digital healthcare.

- Details
- Written by: Centricity Vision
- Category: Press Releases
Centricity Vision Inc., a global ophthalmic technology company, announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the new ZEPTOLink™ IOL Positioning System.
- Company Name: Centricity Vision
- Company/Org Logo:

- About Company: Based in Carlsbad, California, Centricity Vision is a global ophthalmic technology company and developer of the U.S. and internationally approved ZEPTO IOL Positioning System™. Centricity Vision's expert team is dedicated to providing advanced surgical solutions to improve long-term visual outcomes and deliver the best vision care to patients.

- Details
- Written by: Smith & Nephew plc
- Category: Press Releases
Smith+Nephew (LSE: SN, NYSE: SNN), the global medical technology company, today announces its PICO Single Use Negative Pressure Wound Therapy Systems have received an Innovative Technology contract from Vizient, Inc. the nation's largest member-driven health care performance improvement company.
- Company Name: Smith & Nephew plc
- Company/Org Logo:

- About Company: Smith+Nephew is a portfolio medical technology company focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people's bodies and their self-belief by using technology to take the limits off living. We call this purpose 'Life Unlimited'. Our 19,000 employees deliver this mission every day, making a difference to patients' lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.

- Details
- Written by: Olympus
- Category: Press Releases
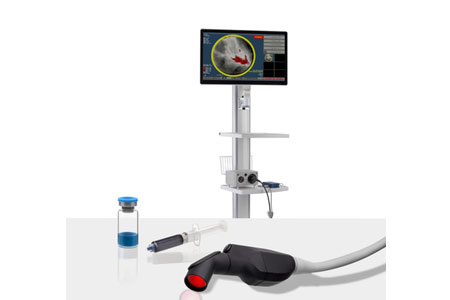
Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, announced the FDA clearance of the new EVIS X1 endoscopy system, along with two compatible gastrointestinal endoscopes.
- Company Name: Olympus
- Company/Org Logo:

- About Company: As a leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients and their safety. Olympus' Medical portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments for endoscopic and therapeutic applications.
- Details
- Written by: Lumicell, Inc.
- Category: Press Releases
Lumicell, Inc., a privately held company focused on innovative fluorescence-guided imaging technologies for cancer surgery, announced that results of the Investigation of Novel Surgical Imaging for Tumor Excision (INSITE).
- Company Name: Lumicell, Inc.
- Company/Org Logo:

- About Company: Lumicell is a privately held company focused on improving surgical outcomes and reducing healthcare costs by utilizing its innovative fluorescence-guided surgical technologies to enable a more complete resection of cancer that may have otherwise been left behind. The company’s first product in development is the Lumicell Direct Visualization System, designed to illuminate residual cancerous tissue within the breast cavity during the initial lumpectomy procedure. Lumicell’s proprietary, pan-oncologic optical imaging agent LUMISIGHT is also being explored across a wide variety of solid tumor indications.
- Details
- Written by: CardieX Limited
- Category: Press Releases
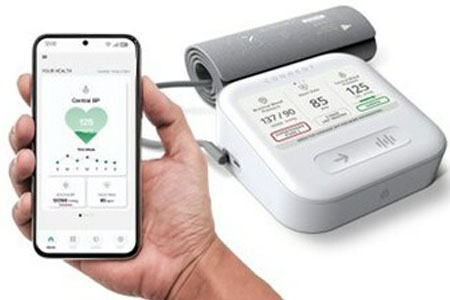
CardieX Limited announced its new arterial health monitor, the CONNEQT Pulse (Pulse), has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pulse is the only vital signs monitor targeted at home, clinician, and clinical trial use that provides measurements of both brachial blood pressure (the pressure at your arm) and central blood pressure (the pressure at your aorta/heart) in addition to multiple other vascular health biomarkers.
- Company Name: CardieX Limited
- Company/Org Logo:

- About Company: CardieX is a health technology company focused on devices & solutions for the world's largest population health disorders. Its ATCOR subsidiary is a world leader in the monitoring of vascular biomarkers for clinical trials and health care research based on the Company's "gold standard" SphygmoCor® central blood pressure technology. CardieX's CONNEQT subsidiary develops and markets medical devices, digital solutions, and wearables for home health, remote patient monitoring, and decentralized ("anywhere") monitoring for clinical trials.
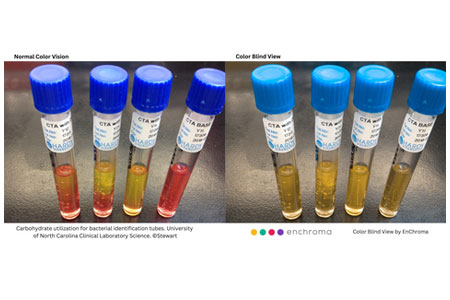
- Details
- Written by: EnChroma
- Category: Press Releases
EnChroma – creators of glasses for color blindness – and the University of North Carolina at Chapel Hill’s Department of Health Sciences announced that special EnChroma glasses for color blindness will be available for students, faculty and staff who are Color Vision Deficient (CVD).
- Company Name: EnChroma
- Company/Org Logo:

- About Company: Based in Berkeley, Calif., EnChroma produces leading-edge eyewear for color blindness and low vision, and other solutions for color vision, sold online and through Authorized Retailers worldwide. Invented in 2010, EnChroma’s patented eyewear combines the latest in color perception, neuroscience and lens innovation to improve the lives of people with color vision deficiency around the world. EnChroma received an SBIR grant from the National Institutes of Health (NIH). It earned the 2016 Tibbetts Award from the U.S. Small Business Administration in recognition of the firm’s innovative impact on the human experience through technology, and the 2020 Innovation Award in Life Sciences from the Bay Area’s East Bay Economic Development Alliance.
- Person of Contact: Mary Catherine Ratliff
- Designation: Director of Communications

- Details
- Written by: Orthofix Medical Inc
- Category: Press Releases
Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, announced the full commercial launch of two access retractor systems to aid surgeons during minimally invasive spine (MIS) procedures.
- Company Name: Orthofix Medical Inc.
- Company/Org Logo:

- About Company: The newly merged Orthofix-SeaSpine organization is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions and a leading surgical navigation system. Its products are distributed in approximately 68 countries worldwide. The Company is headquartered in Lewisville, Texas and has primary offices in Carlsbad, CA, with a focus on spine and biologics product innovation and surgeon education, and Verona, Italy, with an emphasis on product innovation, production, and medical education for orthopedics. The combined company’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France and São Paulo, Brazil.
- Person of Contact: Alexa Huerta
- Designation: Investor Relations
- PlaClin-M, for Safe Environments in the Pandemic Era
- Ancora Heart’s AccuCinch System Demonstrates Significant Improvement in Quality of Life
- TransMed7, LLC Announces First Clinical Use of VacuPac®
- Lumicell Submits New Drug Application for LUMISIGHT™
- Syft Technologies Announces Next Generation SIFT-MS, Syft Tracer™
