Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',

- Details
- Written by: Omnicell, Inc
- Category: Press Releases
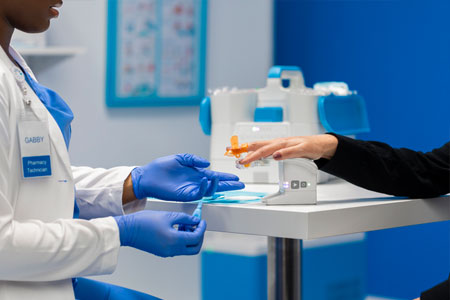
Omnicell, Inc. a leader in transforming the pharmacy care delivery model, announced XT Amplify, a multi-year innovation program that is intended to maximize value for hospitals, health systems, and post-acute care facilities that have invested in Omnicell’s XT Automated Dispensing System while also seeking to drive enhanced clinical and operational outcomes at the points of care and within pharmacies.
- Company Name: Omnicell, Inc
- Company/Org Logo:

- About Company: Since 1992, Omnicell has been committed to transforming pharmacy care through outcomes-centric innovation designed to optimize clinical and business outcomes across all settings of care. Through a comprehensive portfolio of robotics, smart devices, intelligent software, and expert services, Omnicell solutions are helping healthcare facilities worldwide to reduce costs, improve labor efficiency, establish new revenue streams, enhance supply chain control, support compliance, and move closer to the industry vision of the Autonomous Pharmacy.
- Person of Contact: Betsy Martinelli
- Designation: Director
- Details
- Written by: Babson Diagnostics
- Category: Press Releases
Babson Diagnostics, a science-first healthcare technology company,announced the newest innovation in its BetterWay™ blood testing ecosystem has completed validation testing. The company created a unique hand-warming technology specifically designed to support the collection of high-quality blood samples from a fingertip.
- Company Name: Babson Diagnostics
- Company/Org Logo:

- About Company: Babson Diagnostics is a science-first healthcare technology company reimagining the entire diagnostic blood testing experience. Babson's mission is to make routine blood testing less invasive, more convenient, and affordable, empowering people to take charge of their health. Based in Austin, Babson is led by individuals with deep experience in healthcare, diagnostics, engineering, and laboratory technologies. The company is named in honor of Art Babson, whose legacy of scientific innovation and excellence is the foundation on which the company is built.
- Person of Contact: Joe Foster
- Details
- Written by: Centinel Spine, LLC
- Category: Press Releases
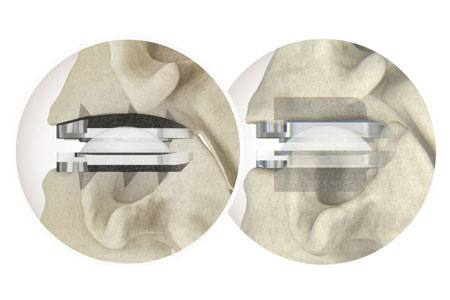
Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), announced the completion of the 5,000th case in the U.S. with the prodisc C Vivo and prodisc C SK Cervical TDR System.
- Company Name: Centinel Spine, LLC
- Company/Org Logo:

- About Company: Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world. The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 250,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
- Person of Contact: Varun Gandhi
- Designation: Chief Financial Officer
- Details
- Written by: 4WEB Medical
- Category: Press Releases
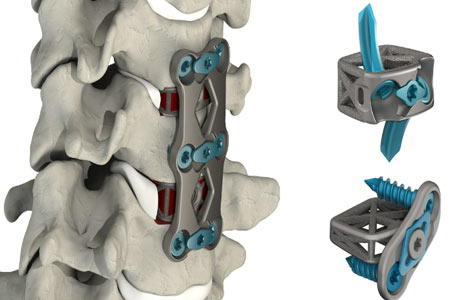
4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced the full commercial launch of the newest addition to the company's implant portfolio, the Cervical Spine Plating Solution (CSTS-PS).
- Company Name: 4WEB Medical
- Company/Org Logo:

- About Company: 4WEB Medical, founded in 2008 in Dallas, TX, is an orthopedic implant company. Thirty years of research in topological dimension theory led to the discovery of a novel geometry, the 4WEB, that can be used as a building block to create high-strength, lightweight web structures. The company leveraged this breakthrough to develop 4WEB Medical's proprietary truss implant platform which was the 1st 510(k) cleared implant manufactured with 3D printing technology. The 4WEB Medical product portfolio includes the Cervical Spine Truss System™, the Stand Alone Cervical Spine Truss System™, the Stand Alone Anterior Spine Truss System, the Anterior Spine Truss System™, the Posterior Spine Truss System™, and the Lateral Spine Truss System™.
- Details
- Written by: Acclaro Medical Corporation
- Category: Press Releases
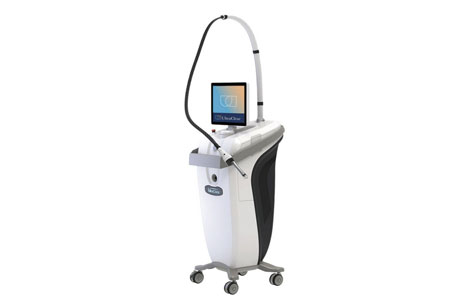
Acclaro Medical proudly announces new clinical study results on nasal contouring, acne scarring, facial resurfacing, facial and neck rejuvenation with stem cell extract and UltraClear® Laser-Coring™ will be presented at the American Society for Laser Medicine and Surgery (ASLMS) global conference, to be held April 11-14, 2024 in Baltimore, MD.
- Company Name: Acclaro Medical Corporation
- Company/Org Logo:

- About Company: Acclaro Medical is a pioneering medical technology company focused on developing cutting-edge solutions that improve patient care and redefine medical practices. With a relentless commitment to innovation and a team of dedicated professionals, Acclaro Medical continues to push the boundaries and drive positive change in the aesthetic medical industry.
- Person of Contact: Nadine Tosk

- Details
- Written by: Smith+Nephew
- Category: Press Releases
Smith+Nephew the global medical technology company, announces the review of medical technologies guidance from the UK National Institute for Health and Care Excellence (NICE) for its PICO Single Use Negative Pressure Wound Therapy System (sNPWT).
- Company Name: Smith+Nephew
- Company/Org Logo:

- About Company: Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management. Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.5 billion in 2023. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
- Person of Contact: Dave Snyder

- Details
- Written by: Medline Industries, LP
- Category: Press Releases
Medline and Consure Medical announced a new agreement for Medline to exclusively distribute the QiVi® MEC male external urine management device to help guard against catheter-associated urinary tract infections (CAUTI) and incontinence-associated dermatitis (IAD).
- Company Name: Medline Industries, LP
- Company/Org Logo:

- About Company: Medline is a healthcare company – a medical supply manufacturer, distributor, and solutions provider focused on improving the overall operating performance of healthcare. Partnering across the continuum of care, Medline helps providers to activate the clinical and supply chain resources needed to deliver their best care. With the agility to solve problems quickly and the scale to partner with providers for their sustained success, Medline is able to invest in its customers for the future and rapidly respond to a dynamically changing market with customized solutions. Medline was most recently named to the Forbes America's Best Large Employers and Best Employers for Women lists, and was recognized for the 13th year by Chicago Tribune as a Top Workplace. Headquartered in Northfield, Ill., Medline has 38,000+ employees worldwide and operates in over 100 countries and territories.

- Details
- Written by: Guardant Health Inc
- Category: Press Releases
Guardant Health Inc, a leading precision oncology company, announced that results from the ECLIPSE study showing the effectiveness of its ShieldTM blood test for detecting colorectal cancer (CRC) in average-risk adults will be published in the March 14 issue of The New England Journal of Medicine.
- Company Name: Guardant Health Inc
- Company/Org Logo:

- About Company: Guardant Health is a leading precision medicine company focused on guarding wellness and giving every person more time free from cancer. Founded in 2012, Guardant is transforming patient care by providing critical insights into what drives disease through its advanced blood and tissue tests, real-world data and AI analytics. Guardant tests help improve outcomes across all stages of care, including screening to find cancer early, monitoring for recurrence in early-stage cancer, and helping doctors select the best treatment for patients with advanced cancer.
- Person of Contact: Michael Weist
- Phone: 650-647-3643
- Details
- Written by: Medline Industries, LP
- Category: Press Releases
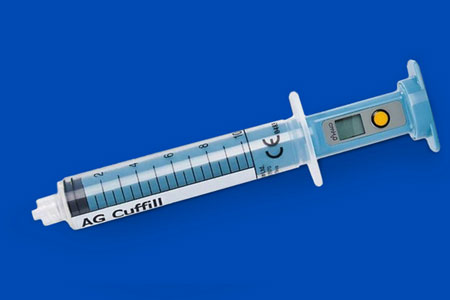
Medline, a market-leading manufacturer and supplier of medical supplies and solutions, announced the successful acquisition of the manufacturing rights and intellectual property of AG Cuffill from Hospitech Respiration Ltd. The compact and intuitive syringe-like device provides an accurate solution for measuring both pressure and volume of airway cuffs in all clinical settings.
- Company Name: Medline Industries, LP
- Company/Org Logo:

- About Company: Medline is a healthcare company – a medical supply manufacturer, distributor, and solutions provider focused on improving the overall operating performance of healthcare. Partnering across the continuum of care, Medline helps providers to activate the clinical and supply chain resources needed to deliver their best care. With the agility to solve problems quickly and the scale to partner with providers for their sustained success, Medline is able to invest in its customers for the future and rapidly respond to a dynamically changing market with customized solutions. Medline was most recently named to the Forbes America's Best Large Employers and America's Best Employers for Women lists, and was recognized for the 13th year by Chicago Tribune as a Top Workplace. Headquartered in Northfield, Ill., Medline has 36,500+ employees worldwide and operates in over 110 countries and territories.

- Details
- Written by: Bruker Corporation
- Category: Press Releases
Bruker Corporation is pleased to announce the successful closing of its acquisition of Chemspeed Technologies AG, a leading Swiss provider of automated laboratory R&D and QC workflow solutions.
- Company Name: Bruker Corporation
- Company/Org Logo:

- About Company: Bruker is enabling scientists to make breakthrough discoveries and develop new applications that improve the quality of human life. Bruker’s high performance scientific instruments and high value analytical and diagnostic solutions enable scientists to explore life and materials at molecular, cellular and microscopic levels. In close cooperation with our customers, Bruker is enabling innovation, improved productivity and customer success in life science molecular and cell biology research, in applied and pharma applications, in microscopy and nanoanalysis, as well as in industrial applications. Bruker offers differentiated, high-value life science and diagnostics systems and solutions in preclinical imaging, clinical phenomics research, proteomics and multiomics, spatial and single-cell biology, functional structural and condensate biology, as well as in clinical microbiology and molecular diagnostics.
- Person of Contact: Justin Ward
- CLEAR GUIDE SCENERGY™ Device for Transperineal Interventions
- Aerin Medical Announces Positive Two-year Outcomes from the VATRAC Trial of VivAer
- ViiV Healthcare announces new packaging option now available in the U.S. for Dovato
- Edwards’ EVOQUE Valve Replacement System First Transcatheter Therapy to Earn FDA Approval for Tricuspid Valve
- PrepMD's online cardiac healthcare training solutions now approved for CEUs
