Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: THINK Surgical, Inc.
- Category: Press Releases
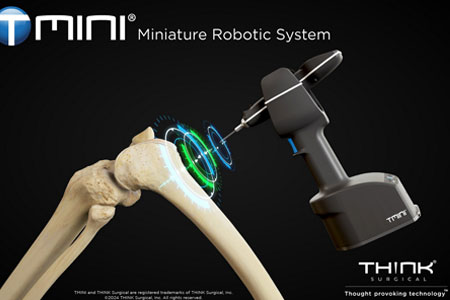
THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, announced that its TMINI® Miniature Robotic System (TMINI 1.1) has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
- Company Name: THINK Surgical, Inc.
- Company/Org Logo:

- About Company: THINK Surgical, Inc., is a privately held U.S.-based technology innovator that develops and markets orthopedic robots. THINK Surgical actively collaborates with healthcare professionals to refine our orthopedic products, improving the lives of those suffering from advanced joint disease with precise, accurate, and intelligent technology. Please refer to the instructions for use for the TMINI Miniature Robotic System for a complete list of indications, contraindications, warnings, and precautions.
- Person of Contact: Nick Margree

- Details
- Written by: Ceribell, Inc.
- Category: Press Releases
Ceribell, Inc., a commercial-stage medical technology company focused on transforming the detection and management of patients with serious neurological conditions, announced the publication of results from a new multi-center retrospective study of patient outcomes following use of the Ceribell system compared to conventional electroencephalography ("EEG").
- Company Name: Ceribell, Inc.
- Company/Org Logo:

- About Company: Ceribell, Inc. is a developer of AI-powered point-of-care electroencephalography (EEG) technology dedicated to improving the detection and treatment of neurological conditions. Our goal is to revolutionize brain health by making EEG diagnostics widely available, more efficient, and more cost-effective. We are initially focused on becoming the standard of care for the detection and management of non-convulsive seizures in the acute care setting. Ceribell is headquartered in Sunnyvale, Calif.
- Person of Contact: Corrie Rose
- Details
- Written by: Vectorious Medical Technologies Ltd.
- Category: Press Releases
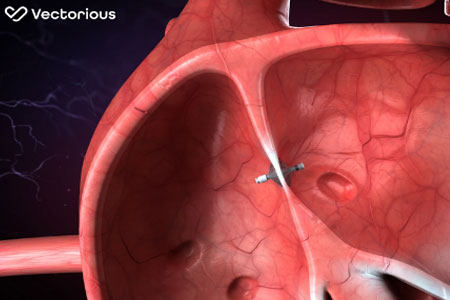
A patient in Cincinnati has become the first in the United States to receive an innovative system that enables remote monitoring of his heart and congestion status, as well as self-adjustment of diuretics.
- Company Name: Vectorious Medical Technologies Ltd.
- Company/Org Logo:

- About Company: Vectorious is a Tel Aviv-based, privately held company, founded in 2011. The company's V-LAP System enables heart failure patients to better control their disease by remotely monitoring the heart's Left Atrial Pressure (LAP) and detecting fluid accumulation in the earliest stages of the disease, prior to physiological symptoms. A patient app further empowers the patient to self-titrate medication in real-time based on data extracted from the heart.
- Person of Contact: Lili Arbely-Elbaz
- Details
- Written by: VisionAir Solutions
- Category: Press Releases
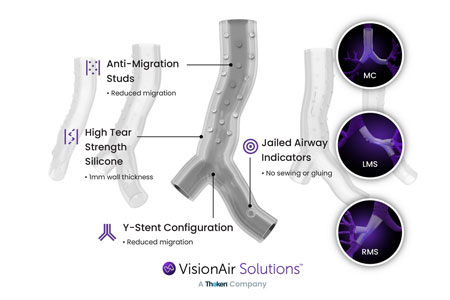
VisionAir Solutions (VAS), the small company with a big idea for better therapeutic solutions, proudly announces a significant milestone of custom developing over 600 patient-specific airway stents using our AI VisionAir 3D Stent Software—helping save the lives of these patients.
- Company Name: VisionAir Solutions
- Company/Org Logo:

- About Company: VisionAir Solutions is a leading provider of innovative medical solutions, specializing in personalized airway stents designed to enhance respiratory function and improve patient quality of life.

- Details
- Written by: NextStep Arthropedix
- Category: Press Releases
NextStep Arthropedix, a growing orthopedic implants and procedures company, announced the launch of TheRay™ Collared and Collarless Hip System.
- Company Name: NextStep Arthropedix
- Company/Org Logo:

- About Company: NextStep Arthropedix is a division of the Akron, Ohio-based company, Theken. Its team is focused on the design and development of joint implants and procedures. The NextStep Arthropedix team combines successful industry experience with fresh ideas and new technologies to create a lasting impact on the lives of its surgeons and their patients. The company incorporates 3D-printing technology in its design and manufacturing processes and has formed several new divisions focused on medical ceramics, antimicrobials, and microelectronics. In 2017, NextStep launched its flagship iNSitu™ Hip Systems and since has added several product lines: iNSitu™ Blade Hip System, iNSitu™ Cemented Stem, iNSitu™ Bipolar Hip System, iNSitu™ Multi-Hole 3D printed cup. In 2023, NextStep launched The Preserve by NextStep, the first retractorless, assistantless, anterior hip approach eliminating soft tissue and bone injury caused by retractors.
- Details
- Written by: Bedal International
- Category: Press Releases
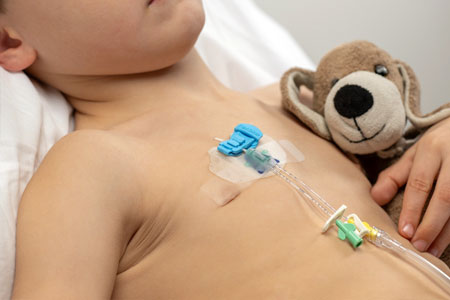
Bedal International, a Belgian based medical device company delivering next generation catheter securement solutions, announces the successful closing of a $11 million funding round. The investment will be used to support the company's ongoing global growth. Bedal has offices in Belgium (EU) and in Anaheim, CA.
- Company Name: Bedal International
- Company/Org Logo:

- About Company: Bedal International, a dynamic scale-up in the medical device industry, focuses on specialized catheter securement solutions. The FlexGRIP® products prioritize patient well-being and healthcare provider convenience. Dedicated to innovation, Bedal International strives to enhance the overall quality of patient care.

- Details
- Written by: Stratasys
- Category: Press Releases
Stratasys Ltd. (NASDAQ: SSYS), announced the launch of the groundbreaking DentaJet™ XL, the latest innovation in dental 3D printing technology. This new high-speed 3D printer is the latest addition to the DentaJet series, designed to further improve dental lab productivity and reduce costs with its larger resin cartridges, large print tray, Super High-Speed mode, and minimal post processing workflow.
- Company Name: Stratasys
- Company/Org Logo:

- About Company: Stratasys is leading the global shift to additive manufacturing with innovative 3D printing solutions for industries such as aerospace, automotive, consumer products and healthcare. Through smart and connected 3D printers, polymer materials, a software ecosystem, and parts on demand, Stratasys solutions deliver competitive advantages at every stage in the product value chain. The world’s leading organizations turn to Stratasys to transform product design, bring agility to manufacturing and supply chains, and improve patient care. To learn more about Stratasys, visit www.stratasys.com, the Stratasys blog, X/Twitter, LinkedIn, or Facebook. Stratasys reserves the right to utilize any of the foregoing social media platforms, including Stratasys’ websites, to share material non-public information pursuant to the SEC’s Regulation FD. To the extent necessary and mandated by applicable law, Stratasys will also include such information in its public disclosure filings.
- Person of Contact: Chris Reese
- Designation: Investor Relations
- Details
- Written by: New4med
- Category: Press Releases
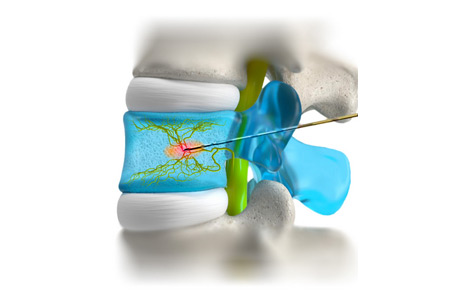
New4med GmbH, a German startup focused on innovative spinal treatments, announces the successful European market launch of its novel Basivertebral Nerve Ablation (BVNA) therapy.
- Company Name: New4med
- Company/Org Logo:

- About Company: New4med GmbH is a german medtech company specializing in the development and distribution of innovative products and procedures in spinal surgery. The product range aims to treat chronic back pain in the spine, improving patients' quality of life sustainably. New4med's experienced team consists of professionals from the medtech industry who bring their expertise and passion for innovation to the development and distribution of their products. New4med prides itself on improving the quality of life for millions of people worldwide.
- Person of Contact: Daniel Seifert
- Details
- Written by: DP Technology
- Category: Press Releases
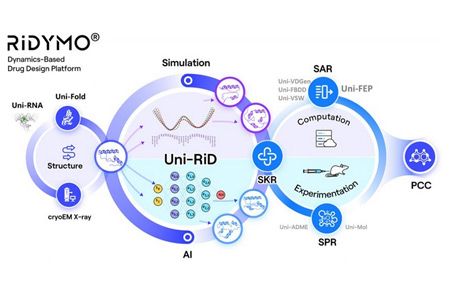
DP Technology, an "AI for Science" paradigm-driven company, today announced the nomination of DPT0416, a novel CNS penetrable small molecule targeting Lp-PLA2, as a preclinical candidate for the treatment of Alzheimer's disease (AD).
- Company Name: DP Technology
- Company/Org Logo:

- About Company: DP Technology, an "AI for Science" new paradigm-driven company, dedicated to applying Artificial Intelligence and Molecular Simulation algorithms to solve important scientific problems by combining advanced computational methods. Relying on DP Technology's Dynamics-based drug design platform, RiDYMO®, we have set up a world-leading hit discovery platform. The team has established external collaborations and built up strong internal pipeline, focusing on three areas of CNS, oncology and autoimmune diseases.
- Person of Contact: Dr. Xiaomin Zhang
- Details
- Written by: Carestream Health
- Category: Press Releases
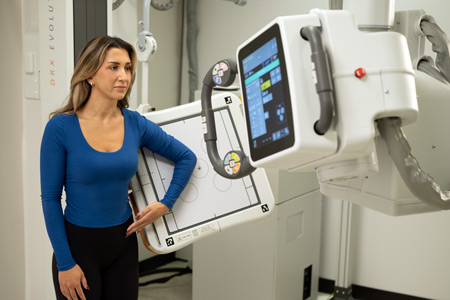
Carestream Health announced the addition of new enhancements to its ImageView Software and DRX-Evolution Plus System, marking the latest improvements to its line of innovative, industry-leading imaging solutions.
- Company Name: Carestream Health
- Company/Org Logo:

- About Company: Carestream is a worldwide provider of medical imaging systems; X-ray imaging systems for non-destructive testing; and precision contract coating services for a wide range of industrial, medical, electronic, and other applications—all backed by a global service and support network.
- Person of Contact: Melody Warner
- American Regent Introduces Sodium Phosphates Injection, USP; FDA-Approved
- Paradromics accepted to FDA regulatory accelerator program and announces new patient registry
- RamSoft and RADPAIR Announce Integration of AI-Driven Radiology Report Generation into OmegaAI's Platform
- Heidelberg University Hospital in Germany Invests in Fourth Accuray Radiotherapy Device, the Radixact® System, to Improve Cancer Patient Care
- GE HealthCare and MediView Announce World's First Installation and Clinical Use of Augmented Reality Interventional Suite to Transform Interventional Radiology
