Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: ovalon Technologies Ltd
- Category: Press Releases
Covalon Technologies Ltd. an advanced medical technologies company, announced its participation in the Infusion Nurses Society Visitors to Covalon’s booth will have the opportunity to explore the Company’s innovative vascular access and infection control solutions first-hand and learn why many top hospitals are choosing Covalon solutions for their patients.
- Company Name: Covalon Technologies Ltd
- About Company: Covalon is a patient-driven medical device company, that provides innovative and cost-effective healthcare solutions for advanced wound care, infection control, and medical device coatings. Through a strong portfolio of patented technologies and solutions, we offer innovative, gentle and more compassionate options to aid patients on their healing journey. Our solutions are designed for patients and made for care providers. Covalon leverages its patented medical technology platforms and expertise in two ways: (i) by developing products that are sold under Covalon’s name; and (ii) by developing and commercializing medical products for other medical companies under development and license contracts. The Company is listed on the TSX Venture Exchange, having the symbol COV and trades on the OTCQX Market under the symbol CVALF.
- Person of Contact: Brian Pedlar
- Details
- Written by: Olympus
- Category: Press Releases
Olympus, a global medical technology company committed to making people's lives healthier, safer and more fulfilling, announced today that it will highlight its EVIS X1™ endoscopy system and colorectal cancer screening solutions.
- Company Name: Olympus
- Company/Org Logo:

- About Company: At Olympus, we are committed to our purpose of making people's lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services that will help them with early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America.

- Details
- Written by: Olympus Corporation of the Americas
- Category: Press Releases
Olympus, a global medical technology company committed to making people's lives healthier, safer and more fulfilling, announced that it will highlight its algorithm-powered emphysema screening program along with two exciting bronchoscopes compatible with the EVIS X1™ endoscopy system as part of the Olympus Hybrid Bronchoscopy.
- Company Name: Olympus Corporation of the Americas
- Company/Org Logo:

- About Company: At Olympus, we are committed to our purpose of making people's lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America.
- Details
- Written by: NEO Semiconductor
- Category: Press Releases
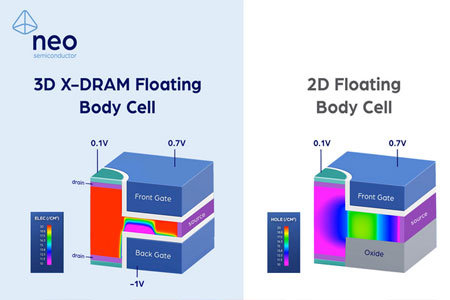
NEO Semiconductor, a leading developer of innovative technologies for 3D NAND flash and DRAM memory, announced a performance boosting Floating Body Cell Mechanism for 3D X-DRAM. Andy Hsu, Founder & CEO presented groundbreaking Technology CAD (TCAD) simulation results for NEO's 3D X-DRAM™.
- Company Name: NEO Semiconductor
- Company/Org Logo:

- About Company: NEO Semiconductor is a high-tech company focused on advancing 3D NAND flash and DRAM technologies. The company was founded in 2012 by Andy Hsu and a team in San Jose, California, and owns more than 24 U.S. patents. In 2020, the company made a breakthrough in 3D NAND architecture named X-NAND™ that can achieve SLC performance from TLC and QLC memory to provide high-speed, low-cost solutions for many applications, including 5G and AI. In 2022, the company launched its X-DRAM™ technology, representing a new architecture that can deliver DRAM with the world's lowest power consumption. In 2023, NEO launched its ground-breaking 3D X-DRAM™ technology, a game changer in the memory industry, enabling the world's first 3D NAND-like DRAM to solve capacity scaling bottlenecks and move the market past the limitations of 2D DRAM.
- Person of Contact: Maya Lustig

- Details
- Written by: Olympus Corporation of the Americas
- Category: Press Releases
Olympus, a global medical technology company, announced two bronchoscopes as part of the EVIS X1 Endoscopy System. The EVIS X1 Endoscopy System represents the latest in diagnostic and therapeutic bronchoscopy from Olympus.
- Company Name: Olympus Corporation of the Americas
- Company/Org Logo:

- About Company: At Olympus, we are committed to our purpose of making people's lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America.
- Details
- Written by: Preeclampsia Foundation
- Category: Press Releases
The U.S. Department of Health and Human Services (HHS) Office on Women's Health has announced that the Preeclampsia Foundation Cuff Kit™ program will be joining 10 other final phase winners for the HHS Hypertension Innovator Award Competition.
- Company Name: Preeclampsia Foundation
- Company/Org Logo:

- About Company: The Preeclampsia Foundation is a U.S.-based 501(c)(3) non-profit organization established in 2000 to improve the outcomes of hypertensive disorders of pregnancy by educating, supporting, and engaging the community, improving healthcare practices, and finding a cure. We envision a world where preeclampsia and related hypertensive disorders of pregnancy no longer threaten the lives of mothers and babies.
- Person of Contact: Laney Poye

- Details
- Written by: Lumenis Be Ltd.
- Category: Press Releases
Lumenis Be Ltd., a leading energy-based medical device company for aesthetic and eye care applications, announced that triLift is the winner of the highly competitive "Best New Dermatology Technology Solution" award in the 8th annual MedTech Breakthrough Awards program.
- Company Name: Lumenis Be Ltd.
- Company/Org Logo:

- About Company: triLift treatment is not suitable for patients with pacemakers, defibrillators, or any implanted electronic devices, as well as metal implants in the treatment area. Risks may include damage to natural skin texture, skin redness, swelling, bruising, itching and change of pigmentation. Be sure to consult with your treatment provider before choosing this treatment.
- Person of Contact: Gabby White

- Details
- Written by: CIONIC
- Category: Press Releases
CIONIC, the neurotech inventor of FDA-cleared bionic clothing to improve walking, strength, and overall mobility, announced significant updates to the Cionic Neural Sleeve experience in its latest software release.
- Company Name: CIONIC
- Company/Org Logo:

- About Company: CIONIC is a neurotech company committed to exceeding the expectations of human capability and changing the lives of people with mobility differences by facilitating more independent movement through FDA-cleared bionic clothing. Motivated by his daughter's journey with cerebral palsy, technology innovator Jeremiah Robison founded CIONIC in 2018. The company’s lead product, the Cionic Neural Sleeve, can analyze and augment human movement, enabling the body to move with more freedom and control than with crutches, walkers, or wheelchairs. The Cionic Neural Sleeve thoughtfully combines the diagnostic power of a gait lab with the therapeutic power of Functional Electrical Stimulation (FES) into a lightweight, durable garment that can be worn anywhere and work everywhere.
- Person of Contact: Christy Maginn
- Details
- Written by: Caregility
- Category: Press Releases
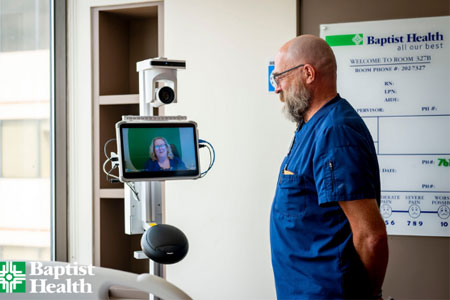
Baptist Health and Caregility are pleased to announce an expanded partnership aimed at enhancing patient care across the Arkansas-based healthcare organization.
- Company Name: Caregility
- Company/Org Logo:

- About Company: Caregility (caregility.com) is a telehealth solution provider connecting care everywhere. Designated as the Best in KLAS® Virtual Care Platform (non-EMR) in 2021, 2022, and 2023, Caregility Cloud™ brings bedside care, virtual encounters, and AI capabilities together at the point of care. Doctors, nurses, and patients around the world rely on our intelligent telehealth edge devices and virtual nursing, observation, and engagement applications to enhance clinical insights, patient safety, and efficiency. Trusted by over 75 health systems, deployed in more than 1,000 hospitals, and supporting over 30,000 connected devices and millions of virtual sessions annually, Caregility is helping transform healthcare delivery across inpatient, outpatient, and home settings.
- Person of Contact: Jess Clifton
- Designation: Senior Manager
- Details
- Written by: iHealth Labs, Inc.
- Category: Press Releases
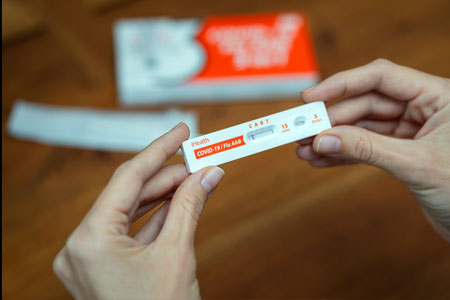
iHealth Labs, Inc. a leading provider of digital health solutions, announced that the U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the company's 3-in-1 COVID-19/Flu A&B Rapid Test Kit.
- Company Name: iHealth Labs, Inc.
- Company/Org Logo:

- About Company: iHealth®, a leading provider of digital health solutions, offers a range of IoT medical devices: blood pressure monitors, glucometers, thermometers, oximeters, and at-home test kits (including its widely-recognized orange-box COVID-19 tests). Since its founding in 2010, the company's consumer-friendly healthcare solutions have been making quality health management more accessible and affordable.
- Lucem Health Unveils Reveal for Lung Cancer: AI-Driven Solution Expedites Screening for At-Risk Patients
- MMI Successfully Concludes First Symani® Clinical Cases in the U.S. Post FDA Commercial Authorization
- CLEW Widens Intelligent Clinical Surveillance Platform to All Inpatient Settings
- 4WEB Medical Announces Regulatory Clearance of its Anterior Spine Truss System with Anchor Fixation
- Universal Robots announces seamless integration with Siemens PLCs
