Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',

- Details
- Written by: GT Metabolic Solutions
- Category: Press Releases
GT Metabolic™ Solutions, Inc. is pleased to announce the completion of the first magnetic compression anastomosis cases in the U.S. Helmuth Billy, MD, of Ventura Advanced Surgical Associates in Ventura, CA, completed the cases using the MagDI™ System.
- Company Name: GT Metabolic Solutions
- Company/Org Logo:

- About Company: GT Metabolic Solutions Inc. is leading the world with its development of magnetic compression anastomosis technologies for next-stage minimally invasive procedures. An elegant approach that uses magnets to help achieve anastomosis, the incisionless, sutureless, staple-free technique leaves no foreign material to impede the natural tissue healing process. Working in tandem with renowned global experts, our team has engineered a magnetic compression solution called delayed anastomosis technology (DAT) that surgeons can use to create consistent anastomosis while helping minimize potential complications, such as leaks and bleeds, in challenging applications. Our solution democratizes the surgical approach to anastomosis. It can be used in procedure staging and is 100% reversible. Committed to improving patients' lives and healthcare provider outcomes, GT Metabolic Solutions Inc. is disrupting the market by introducing magnetic

- Details
- Written by: OMRON Healthcare
- Category: Press Releases
In a pivotal stride to address the growing AFib epidemic, OMRON Healthcare announced the U.S. Food and Drug Administration (FDA) has granted the company its De Novo authorization to market new home blood pressure monitors featuring breakthrough AI-powered atrial fibrillation detection. In a medical device first, OMRON's novel machine learning IntelliSense™ AFib algorithm automatically analyzes the Pressure Pulse Wave generated during blood pressure measurement to detect AFib, a leading cause of stroke1.
- Company Name: OMRON Healthcare
- Company/Org Logo:

- About Company: OMRON Healthcare, Inc., is the world's leading manufacturer and distributor of personal heart health products and an innovator in technologies supporting respiratory and pain management care. With over 50 years of medical device category leadership, OMRON is passionate about empowering people to take charge of their health at home through precise technology. Its market-leading products include a full range of home blood pressure monitors, nebulizers and TENS devices. The company's mission is Going for Zero, the elimination of heart attacks and strokes. With more than 350 million devices sold globally, OMRON provides the world's most recommended blood pressure monitors by healthcare professionals. OMRON Healthcare strives to improve lives and contribute to a better society by developing innovations that help people prevent, treat, and manage their medical conditions. The company provides products and services in over 130 countries.

- Details
- Written by: Johnson & Johnson MedTech
- Category: Press Releases
Shockwave Medical, Inc., part of Johnson & Johnson MedTech and a global leader in the field of circulatory restoration, announced the first clinical outcomes associated with the Shockwave Javelin Peripheral IVL Catheter, a novel, non-balloon-based lithotripsy platform designed to modify calcium and cross extremely narrowed vessels in patients with peripheral artery disease (PAD).
- Company Name: Johnson & Johnson MedTech
- Company/Org Logo:

- About Company: At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity.
- Person of Contact: Rachael Jarnagin
- Details
- Written by: Medtronic plc
- Category: Press Releases
Medtronic plc a global leader in healthcare technology, announced United States Food and Drug Administration (FDA) approval of the Affera™ Mapping and Ablation System with Sphere-9™ Catheter, an all-in-one, high-density (HD) mapping and pulsed field (PF) and radiofrequency (RF) ablation catheter for treatment of persistent atrial fibrillation (AFib) and for RF ablation of cavotricuspid isthmus (CTI) dependent atrial flutter.
- Company Name: Medtronic plc
- Company/Org Logo:

- About Company: Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Galway, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary.
- Person of Contact: Leslie Williamson

- Details
- Written by: Okomera
- Category: Press Releases
Okomera announces Bpifrance funding to develop and standardize novel CRISPR screening assay to accelerate drug discovery in oncology with leading cancer research center of Marseille, CRCM.
- Company Name: Okomera
- Company/Org Logo:

- About Company: Paoli-Calmettes Institute: Based in Marseilles, Paoli-Calmettes Institute (IPC) is the first cancer comprehensive centre in region providing global care for cancer.
- Person of Contact: Sidarth Radjou

- Details
- Written by: HEMOSONICS LLC
- Category: Press Releases
HemoSonics, LLC, a medical device company focused on acute bleeding management, announced it has been awarded one of the $525,000 grand prizes in the National Institutes of Health (NIH) RADx Tech for Maternal Health Challenge. NIH's RADx Tech for Maternal Health Challenge sought to accelerate the development of innovative diagnostic tools and solutions to address the maternal mortality crisis.
- Company Name: HemoSonics
- Company/Org Logo:

- About Company: HemoSonics, LLC is a medical device technology company focused on acute bleeding management, resulting in better patient care and lower overall medical costs. The Quantra Hemostasis Analyzer, HemoSonics' flagship product, is designed to improve patient outcomes and reduce healthcare costs by providing optimized coagulation information. The Quantra System's easy and fast interpretation enables simple, more efficient point-of-care and laboratory bleeding management.

- Details
- Written by: Carl Zeiss Meditec AG
- Category: Press Releases
ZEISS Medical Technology announced the broad U.S. distribution of the MICOR® 700 from ZEISS, reinventing lens extraction with the first hand-held lens removal device with ultrasound-free operation, providing a sustainable solution with a low initial investment to help surgeons broaden their intraocular working space.
- Company Name: Carl Zeiss Meditec AG
- Company/Org Logo:

- About Company: Carl Zeiss Meditec AG which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 4,823 employees worldwide, the Group generated revenue of €2,089.3m in fiscal year 2022/23 (to 30 September). The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China.
- Details
- Written by: Centinel Spine, LLC
- Category: Press Releases
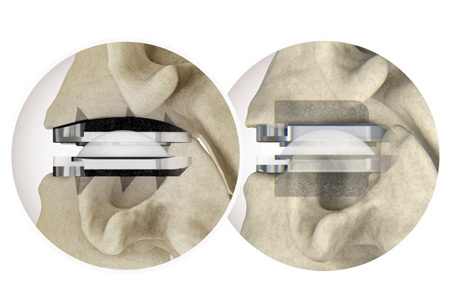
Centinel Spine®, LLC the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), announced the completion of 7,500 U.S. cases with the prodisc C Vivo and prodisc C SK Cervical TDR System since the limited commercial launch two years ago, in September 2022.
- Company Name: Centinel Spine, LLC
- Company/Org Logo:

- About Company: Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world. The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 250,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
- Person of Contact: Varun Gandhi
- Details
- Written by: BD (Becton, Dickinson and Company)
- Category: Press Releases
BD a leading global medical technology company, announced new data that highlights how its advanced artificial intelligence technology can help health systems better identify incidents of controlled substance diversion in the operating room.
- Company Name: BD (Becton, Dickinson and Company)
- Company/Org Logo:

- About Company: BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies.
- Details
- Written by: OrtoWay-US Inc
- Category: Press Releases
OrtoWay-US Inc., inventors and developers of the world’s first hydraulically-powered OrtoWell® Distractor for spinal surgery, announced the receipt of a new patent from the U.S. Patent Office for its OrtoWell® Distractor system. The patent, numbered US 12,023017 B1, entitled “Apparatus, Methods and Systems for Spine Surgery,” was issued on July 2, 2024. It covers the innovative application of the company’s hydraulically powered distractor for use in corpectomy procedures.
- Company Name: OrtoWay-US Inc.
- Company/Org Logo:

- About Company: OrtoWay was founded in 2006 by a group of Swedish experts in biomaterials, spinal surgery and medical technology aiming to enhance anterior surgical techniques and the implantation of spinal prosthesis in the lumbar region. OrtoWay-US Inc., the current developer and IP holder of the OrtoWell® Distractor System is a privately held medical technology company actively seeking commercialization partners. The OrtoWell® instrument recently received a U.S. patent for corpectomy use and is FDA approved for use as a Class 1 Medical Device. The product will be made available through OrtoWay LLC, an independent company based near Philadelphia, Pennsylvania, USA.
- Person of Contact: Stan Mikulowski
- BD expands capacity for advanced prefillable syringes, improving the injection experience for next-generation biologics
- Epitomee Medical announces FDA clearance for its capsule-based weight management device.
- SamanTree Medical receives FDA clearance for the Histolog® Scanner, marking a new era in tissue imaging in the U.S
- Researchers will monitor astronaut brain health using images from the Hyperfine, Inc. Swoop® Portable MR Imaging® System
- Thermo Fisher Scientific announces Oncomine Clinical Research Grant awardees, including the first recipients in India and the Netherlands
