Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: MEDIA CYBERNETICS, INC
- Category: Press Releases
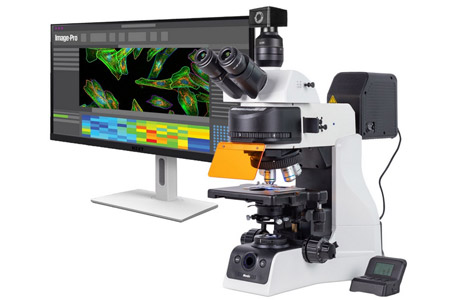
Motic™ Instruments Inc., a global leader in microscopy solutions, and Media Cybernetics Inc., an innovator in AI-powered image analysis software, announce a strategic partnership to deliver cutting-edge microscopy systems that accelerate discovery. This collaboration integrates Motic's advanced microscope systems with Media Cybernetics' Image-Pro® software, providing end-to-end imaging solutions for biological, clinical, research, and materials science applications.
- Company Name: MEDIA CYBERNETICS, INC
- Company/Org Logo:

- About Company: Media Cybernetics, a global leader in image analysis software, powers scientific and industrial research. With decades of expertise, its user-friendly Image-Pro® platform streamlines image capture, processing, and reporting. Media Cybernetics drives innovation, customer success, and strategic partnerships that enable meaningful progress.
- Details
- Written by: Carl Zeiss Meditec AG
- Category: Press Releases
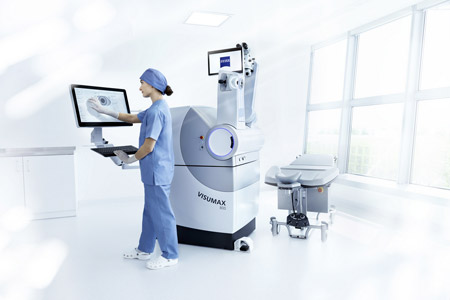
ZEISS Medical Technology announced that the National Medical Products Administration (NMPA) in China has approved the VISUMAX® 800 with SMILE® pro software from ZEISS for surgically treating nearsightedness, with or without astigmatism.
- Company Name: Carl Zeiss Meditec AG
- Company/Org Logo:

- About Company: The Medical Technology segment of ZEISS offers complete solutions to diagnose and treat ophthalmic diseases. In the field of microsurgery the company provides innovative visualization solutions. With a special focus on ophthalmology, ophthalmic surgery and visualization systems in the field of microsurgery, the company supports healthcare professionals in setting new standards of care with proven medical technology and broad application competence based on cutting-edge innovations. ZEISS also offers visualization solutions for dental treatment and gynecology. The company's medical technology portfolio is completed by future-oriented technologies such as intraoperative radiotherapy.
- Details
- Written by: Abanza, Inc.
- Category: Press Releases
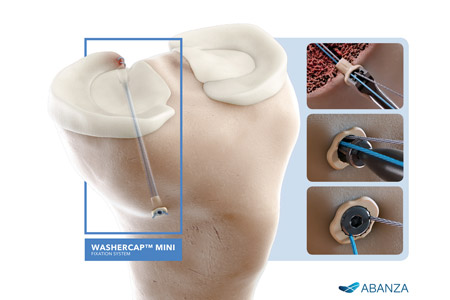
ABANZA, a leader in advanced soft tissue repair solutions, is proud to announce that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its innovative WasherCap™ Mini fixation system. This breakthrough device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
- Company Name: Abanza, Inc.
- Company/Org Logo:

- About Company: At ABANZA, we are revolutionizing orthopedics and arthroscopic soft tissue repair through groundbreaking innovations. Our cutting-edge medical devices are designed to enhance patient outcomes and improve quality of life by empowering surgeons with next-generation solutions in sports medicine and soft tissue fixation. Driven by a commitment to continuous improvement and clinical excellence, ABANZA is at the forefront of advancing medical technology. We are passionate about transforming patient care and setting new industry standards.
- Details
- Written by: Tandem Diabetes Care, Inc
- Category: Press Releases
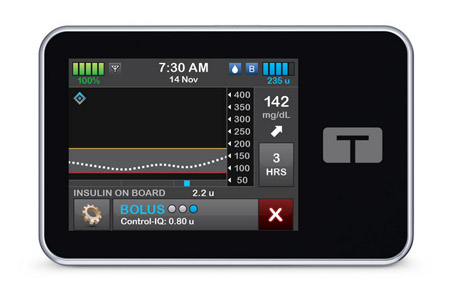
Tandem Diabetes Care, Inc. a leading insulin delivery and diabetes technology company, announced that its next-generation automated insulin delivery (AID) algorithm, Control-IQ+ technology (Control-IQ+), has been cleared by the United State.
- Company Name: Tandem Diabetes Care, Inc.
- Company/Org Logo:

- About Company: Tandem Diabetes Care, a global insulin delivery and diabetes technology company, manufactures and sells advanced automated insulin delivery systems that reduce the burden of diabetes management, while creating new possibilities for patients, their loved ones, and healthcare providers. The Company’s pump portfolio features the Tandem Mobi system and the t:slim X2 insulin pump, both of which feature Control-IQ advanced hybrid closed-loop technology. Tandem Diabetes Care is based in San Diego, California.

- Details
- Written by: VetSnap
- Category: Press Releases
VetSnap, the leading digital logbook solution for controlled drug compliance, and Vaultek, a premier manufacturer of high-performance smart safes, have announced a partnership for hardware and logbook integration that enhances security and streamlines compliance processes for veterinary practices handling controlled substances.
- Company Name: VetSnap
- Company/Org Logo:

- About Company: Founded in 2020, VetSnap is the first DEA-compliant digital controlled drug log software designed specifically for veterinary practices. Built to meet federal regulations and minimum record-keeping requirements in all 50 states, VetSnap streamlines controlled substance management with automated error checking, real-time reconciliation, and secure digital logs. By eliminating manual processes and reducing compliance risks, VetSnap helps veterinary teams save time, ensure accuracy, and maintain audit-ready records effortlessly. Trusted by hospitals and clinics nationwide, VetSnap is transforming controlled drug management with innovative automation and seamless integrations.

- Details
- Written by: Bodybio
- Category: Press Releases
BodyBio, a leader in cellular health solutions, announced the launch of ReMineralize, an innovative ionic mineral supplement designed to optimize cellular function through comprehensive mineral replenishment. This groundbreaking product delivers over 72 naturally occurring trace minerals in their most bioavailable form, setting a new standard in mineral supplementation.
- Company Name: Bodybio
- Company/Org Logo:

- About Company: At BodyBio, we empower health explorers to uncover the root causes of their wellness challenges. It starts with healing the body at its cellular core. As a third-generation family business, we're trusted by over 35,000 doctors. We combine curiosity, authenticity, and empathy to help you discover transformative health solutions. We're more than a supplement company. We're your partner, guiding you through a world of optimal health possibilities.

- Details
- Written by: BD (Becton, Dickinson and Company)
- Category: Press Releases
BD (Becton, a leading global medical technology company, and Biosero, a developer of laboratory automation solutions to orchestrate scientific discoveries, announced a framework collaboration agreement to enable and facilitate robotic arm integration with BD flow cytometry instruments to accelerate drug discovery and development.
- Company Name: BD (Becton, Dickinson and Company)
- Company/Org Logo:

- About Company: BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safet
- Details
- Written by: Lunit
- Category: Press Releases
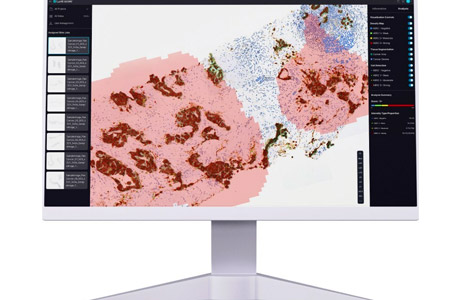
Lunit a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announced the publication of a new study in the Journal of Clinical Oncology Precision Oncology. Conducted in collaboration with Japan's National Cancer Center Hospital East (NCCHE).
- Company Name: Lunit
- Company/Org Logo:

- About Company: Founded in 2013, Lunit (KRX:328130.KQ) is a medical AI company on a mission to conquer cancer. Lunit harnesses AI-powered medical image analytics and biomarker analysis to ensure accurate diagnosis and optimal treatment for each cancer patient. The FDA-cleared Lunit INSIGHT suite for cancer screening serves over 4,500 medical institutions across 55+ countries. Lunit clinical studies have been published in top journals, including the Journal of Clinical Oncology and the Lancet Digital Health, and presented at global conferences such as the ASCO and RSNA. In 2024, Lunit acquired Volpara Health Technologies, setting the stage for unparalleled synergy and accuracy, particularly in breast health and screening technologies. Headquartered in Seoul, South Korea, with a network of offices worldwide, Lunit leads the global fight against cancer.

- Details
- Written by: Centinel Spine, LLC
- Category: Press Releases
Centinel Spine®, LLC the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced achievement of record fourth quarter and full-year 2024 business results, positioning the Company for continued accelerated growth in 2025.
- Company Name: Centinel Spine, LLC
- Company/Org Logo:

- About Company: Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world. The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 275,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
- Details
- Written by: CVS Health
- Category: Press Releases
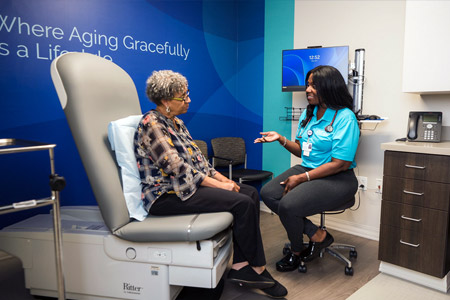
The CVS Health® Foundation announced $4 million in grants over five years as part of its new Healthy Aging initiative to support Atlanta Regional Collaborative for Health Improvement in Atlanta, GA, Center for Better Aging in Chicago, IL, EngageWell.
- Company Name: CVS Health
- Company/Org Logo:

- About Company: CVS Health® is the leading health solutions company, delivering care like no one else can. We reach more people and improve the health of communities across America through our local presence, digital channels and over 300,000 dedicated colleagues — including more than 40,000 physicians, pharmacists, nurses and nurse practitioners. Wherever and whenever people need us, we help them with their health — whether that's managing chronic diseases, staying compliant with their medications or accessing affordable health and wellness services in the most convenient ways. We help people navigate the health care system — and their personal health care — by improving access, lowering costs and being a trusted partner for every meaningful moment of health. And we do it all with heart, each and every day. Follow CVSHealth on social media.
- Person of Contact: Courtney Tavener
- ZEISS MEL 90 excimer laser completes Corneal Refractive Workflow and receives U.S. FDA approval
- Aptiva® Antiphospholipid Syndrome reagents from Werfen receive US FDA 510(k) clearance
- To advance AI-powered real-world health intelligence, RespondHealth collaborates with Microsoft
- A landmark study validates Onera's patch-based home polysomnography system in one of the decade's largest sleep diagnostic trials
- Lunit and Volpara debut a unified ecosystem at aiming to revolutionize global cancer care through integrated technology
