Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',

- Details
- Written by: Exactech, Inc
- Category: Press Releases
Exactech, a global medical technology leader, announced that the U.S. Patent and Trademark Office (USPTO) has issued U.S. patent 12,239,384, further expanding its intellectual property portfolio around the Newton® soft tissue balancing technology. This newly granted patent originates from a series of dedicated patent applications that enable the software-driven evolution of the Newton technology, marking a significant advancement toward autonomous, personalized surgical planning.
- Company Name: Exactech, Inc
- Company/Org Logo:

- About Company: Exactech is a global medical technology leader that empowers orthopaedic surgeons with innovative implants, surgical instruments and the Active Intelligence® (AI) ecosystem of smart technologies to give patients EXACTLY what they need to regain mobility.
- Details
- Written by: Avatar Medical
- Category: Press Releases
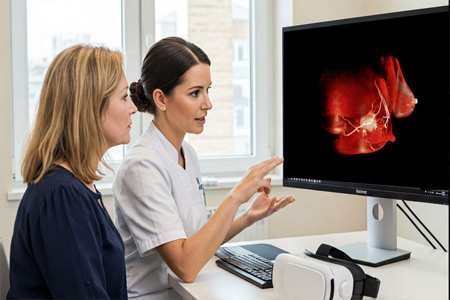
Avatar Medical, a leader in immersive medical imaging, announces that it has obtained CE marking for its innovative solution, Avatar Medical Vision*. This certification marks a decisive step in the company's mission to democratize access to medical images for both surgeons and patients. Avatar Medical Vision enables the real-time conversion of patient medical images into stunning 3D and XR renderings, enhancing visualization and understanding.
- Company Name: Avatar Medical
- Company/Org Logo:

- About Company: Founded in 2020 as a spin-off from the Institut Pasteur and Institut Curie, Avatar Medical democratizes access to medical imaging through advanced 3D visualization software. With offices in Mountain View (California, USA), Paris and Saint-Malo (France), Avatar Medical redefines patient-doctor consultations by enhancing understanding, reducing anxiety, and improving decision-making.
- Details
- Written by: Moon Surgical
- Category: Press Releases
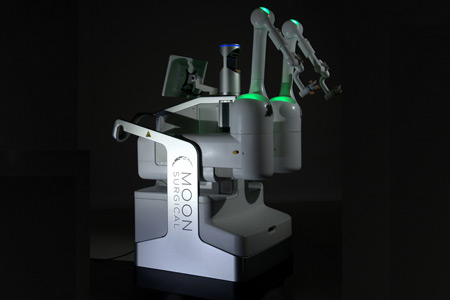
Moon Surgical, a French-American pioneer in surgical innovation, announced it is expanding its long-standing collaboration with NVIDIA to further advance the Maestro System's Physical AI capabilities through NVIDIA Isaac for Healthcare platform.
- Company Name: Moon Surgical
- Company/Org Logo:

- About Company: Moon Surgical, based in Paris, France, and San Francisco, California, is building the OR of the future, one that is digitalized, efficient, and sustainable. The combined power of the transformative Maestro System and the intelligent Maestro Insights empowers healthcare teams to make confident decisions and provide better surgical care for their patients. Founded in 2020, Moon Surgical prides itself on staying nimble, prioritizing innovation, and fostering inclusivity, creativity, and collaboration among its multi-cultural team members.

- Details
- Written by: Silicon Labs
- Category: Press Releases
Silicon Labs the leading innovator in low-power wireless, announced its new Series 2 BG29 family of wireless SoCs, designed to bring high compute and connectivity to the smallest form factor Bluetooth Low Energy (LE) devices without compromising performance. BG29 is ideal for today's most intensive Bluetooth LE applications like wearable health and medical devices, asset trackers, and battery-powered sensors.
- Company Name: Silicon Labs
- Company/Org Logo:

- About Company: Silicon Labs (NASDAQ: SLAB) is the leading innovator in low-power wireless connectivity, building embedded technology that connects devices and improves lives. Merging cutting-edge technology into the world's most highly integrated SoCs, Silicon Labs provides device makers with the solutions, support, and ecosystems needed to create advanced edge connectivity applications. Headquartered in Austin, Texas, Silicon Labs has operations in over 16 countries and is the trusted partner for innovative solutions in the smart home, industrial IoT, and smart cities markets.
- Details
- Written by: GT Metabolic Solution
- Category: Press Releases
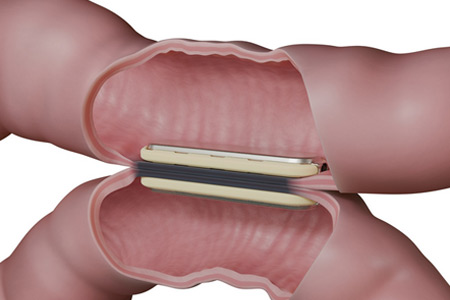
GT Metabolic™ Solutions, Inc., the world leader in magnetic surgery is pleased to announce that the U.S. Food and Drug Administration (FDA) has cleared a larger, 50mm MagDI™ System magnet1 to complement the system's existing 40mm magnet.2 This expansion enables surgeons to accommodate a broader range of patients in magnet-assisted surgery for duodenal-ileal anastomosis. The clearance also underscores GT Metabolic's commitment to driving innovative surgical solutions for improved patient outcomes.
- Company Name: GT Metabolic
- Company/Org Logo:

- About Company: GT Metabolic Solutions Inc. is leading the world with its development of magnetic compression anastomosis technologies for next-stage minimally invasive procedures. An elegant approach that uses magnets to help achieve anastomosis, the incisionless, sutureless, staple-free technique leaves no foreign material to impede the natural tissue healing process.1-5 Working in tandem with renowned global experts, our team has engineered a magnetic compression solution called delayed anastomosis technology (DAT) that surgeons can use to create consistent anastomosis while helping minimize potential complications, such as leaks and bleeds, in challenging applications. Our solution democratizes the surgical approach to anastomosis. It can be used in procedure staging and is 100% reversible. Committed to improving patients' lives and healthcare provider outcomes, GT Metabolic Solutions Inc. is disrupting the market by introducing magnetic compression anastomosis technologies to bariatric
- Person of Contact: Ronald Galovich
- Details
- Written by: Lunit
- Category: Press Releases
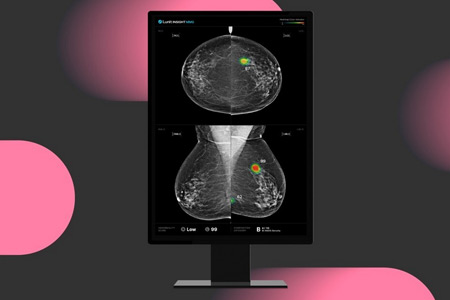
Lunit a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, announced the publication of a groundbreaking prospective study in Nature Communications validating the real-world effectiveness of AI-powered mammography screening in South Korea's national breast cancer screening program.
- Company Name: Lunit
- Company/Org Logo:

- About Company: Founded in 2013, Lunit (KRX:328130.KQ) is a medical AI company on a mission to conquer cancer. Lunit harnesses AI-powered medical image analytics and biomarker analysis to ensure accurate diagnosis and optimal treatment for each cancer patient. The FDA-cleared Lunit INSIGHT suite for cancer screening serves over 4,800 medical institutions across 55+ countries. Lunit clinical studies have been published in top journals, including the Journal of Clinical Oncology and the Lancet Digital Health, and presented at global conferences such as the ASCO and RSNA. Headquartered in Seoul, South Korea, with a network of offices worldwide, Lunit leads the global fight against cancer.
- Details
- Written by: Sky Medical Technology
- Category: Press Releases
Global medical device manufacturer, Sky Medical Technology (Sky), parent company of Firstkind Ltd, announced a limited distribution agreement with SMS Medical, LLC (SMS) – a market leader in orthopaedic device solutions in the Southeast United States. SMS will sell Sky's wearable muscle pump activator, the geko device in the orthopaedic community for post-operative and trauma-based edema (swelling), and for the prevention of DVT (blood clots).
- Company Name: Sky Medical Technology
- Company/Org Logo:

- About Company: Sky Medical Technology (Sky) is a UK-based medical devices company. Through its innovative mechanism of non-invasive neuromuscular electrostimulation (NMES), Sky has developed a ground-breaking NMES technology platform, OnPulse™, embedded in its industry-leading product, the geko™ device – a muscle pump activator. The company develops a range of products tailored to the needs of different medical application areas, selling both direct and through strategic partnerships or distributors in each major clinical area. Clinical areas of interest include the management of trauma-based and orthopaedic pre- and post-operative edema (swelling), the prevention of venous thromboembolism prevention (VTE) and the acceleration of chronic wound healing (leg ulcers). The goal in each therapy area is to partner with healthcare professionals to improve clinical outcomes and patient care while at the same time reducing cost for health systems.
- Details
- Written by: BB Imaging
- Category: Press Releases
BB Imaging, a leading sonography services company, announced its goal to reach 10 million patients annually in the next 10 years. Building on its legacy of serving half a million patients, the company is determined to address the urgent need for improved maternal healthcare and combat the maternal mortality crisis, underscored by the United States' D-plus preterm birth grade from the March of Dimes and Texas' D grade.
- Company Name: BB Imaging
- Company/Org Logo:

- About Company: BB Imaging was founded in 2005 to improve health outcomes by making high-quality ultrasound services accessible. We offer full-service onsite support as well as telesonography®️ services through our proprietary FDA-cleared technology, TeleScan®️. BB Imaging partners with healthcare providers across the U.S. to serve patients where they are, in both urban and rural locations. We are also committed to providing a team-centered working environment prioritizing innovation, excellence, and growth
- Details
- Written by: Olympus Corporation of the Americas
- Category: Press Releases
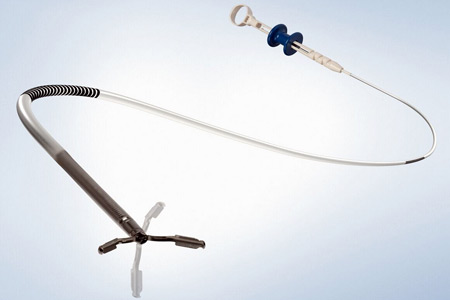
Olympus, a global medical technology company committed to making people's lives healthier, safer and more fulfilling, announced a new hemostasis clip to help meet the needs of GI endoscopists.
- Company Name: Olympus Corporation
- Company/Org Logo:

- About Company: As a leading medical technology company, Olympus uses medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures with the goal of improving clinical outcomes, reducing overall costs, and enhancing the quality of life for patients. Olympus' Medical portfolio includes, but is not limited to, endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of EndoTherapy devices.

- Details
- Written by: Nikon Instruments Inc
- Category: Press Releases
Nikon Instruments Inc. announced the opening of the newest Nikon Center of Excellence at the Moffitt Cancer Center. This prestigious designation underscores Nikon's commitment to providing cutting-edge imaging technology directly to healthcare facilities such as Moffitt Cancer Center for the purpose of developing better cancer treatments and cures.
- Company Name: Nikon Instruments Inc
- Company/Org Logo:

- About Company: Nikon Instruments Inc. is the US microscopy arm of Nikon Healthcare, a world leader in the development and manufacturing of optical, digital imaging technology and software for biomedical applications.
- Motic Instruments and Media Cybernetics partner to deliver application-specific, AI-powered turnkey microscopy systems
- ZEISS VISUMAX 800 with SMILE Pro software receives approval in China
- FDA 510(k) Clearance for ABANZA's WasherCap™ Mini Implantable Fixation Device is received
- Tandem Diabetes Care announces FDA clearance of Control-IQ+ automated insulin delivery technology for people with type 2 diabetes
- VetSnap and Vaultek team up to simplify compliance and strengthen drug security
