Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: ZAP Surgical Systems, Inc.
- Category: Press Releases
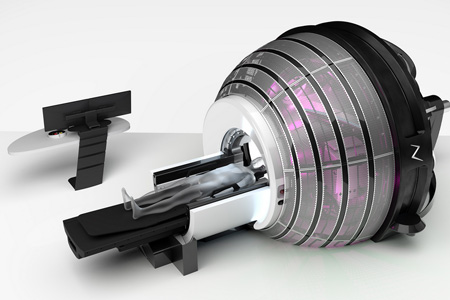
ZAP Surgical Systems, Inc., a global leader in non-invasive robotic brain surgery, announced that its first ZAP-X® Gyroscopic Radiosurgery® platform will soon be installed in Mexico at the newly established Gray Medical Institute, located in San Pedro, part of the Monterrey Metropolitan area in the Mexican state of Nuevo León.
- Company Name: ZAP Surgical Systems, Inc.
- Company/Org Logo:

- About Company: ZAP Surgical Systems, Inc. designs and manufactures the ZAP-X® Gyroscopic Radiosurgery® platform. ZAP was founded in 2014 by Dr. John R. Adler, Emeritus Dorothy & TK Chan Professor of Neurosurgery and Radiation Oncology at Stanford University. Dr. Adler is also renowned as the inventor of the CyberKnife® system and founder of Accuray, Inc. The ZAP-X platform incorporates a unique vault-free design that typically eliminates the need for costly shielded treatment rooms. ZAP-X also utilizes a modern linear accelerator to eliminate legacy use of Cobalt-60.
- Person of Contact: Mark Arnold
- Designation: Senior Vice President, Marketing
- Details
- Written by: Hyperfine, Inc.
- Category: Press Releases
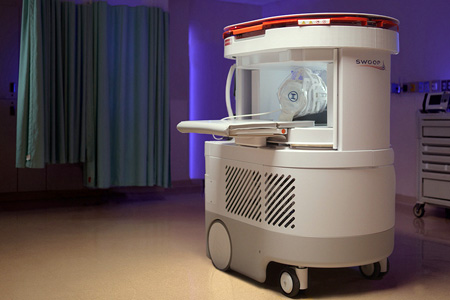
Hyperfine, Inc. the groundbreaking health technology company that has redefined brain imaging with the first FDA-cleared AI-powered portable MRI system for the brain—the Swoop® system—announced FDA clearance of its most significant technological advancement to date. The clearance includes an entirely new portable MRI scanner powered by the proprietary Optive AI™ software.
- Company Name: Hyperfine, Inc.
- Company/Org Logo:

- About Company: Hyperfine, Inc. (Nasdaq: HYPR) is the groundbreaking health technology company that has redefined brain imaging with the Swoop® system—the first FDA-cleared, portable, ultra-low-field, magnetic resonance brain imaging system capable of providing imaging at multiple points of professional care. The mission of Hyperfine, Inc. is to revolutionize patient care globally through transformational, accessible, clinically relevant diagnostic imaging. Founded by Dr. Jonathan Rothberg in a technology-based incubator called 4Catalyzer, Hyperfine, Inc. scientists, engineers, and physicists developed the Swoop® system out of a passion for redefining brain imaging methodology and how clinicians can apply accessible diagnostic imaging to patient care.
- Person of Contact: Devin Zell
- Details
- Written by: GE HealthCare
- Category: Press Releases
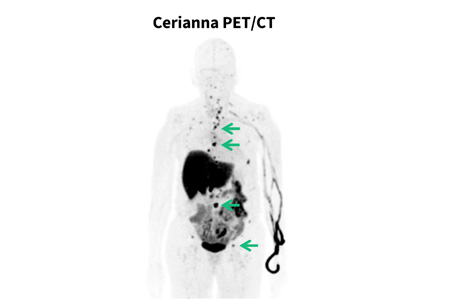
GE HealthCare announced that the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for clinicians now recommend considering fluoroestradiol (FES) positron emission tomography (PET) for systemic staging in patients with recurrent or metastatic lobular breast cancer.
- Company Name: GE HealthCare
- Company/Org Logo:

- About Company: GE HealthCare is a trusted partner and leading global healthcare solutions provider, innovating medical technology, pharmaceutical diagnostics, and integrated, cloud-first AI-enabled solutions, services and data analytics. We aim to make hospitals and health systems more efficient, clinicians more effective, therapies more precise, and patients healthier and happier. Serving patients and providers for more than 125 years, GE HealthCare is advancing personalized, connected and compassionate care, while simplifying the patient’s journey across care pathways. Together, our Imaging, Advanced Visualization Solutions, Patient Care Solutions and Pharmaceutical Diagnostics businesses help improve patient care from screening and diagnosis to therapy and monitoring. We are a $19.7 billion business with approximately 53,000 colleagues working to create a world where healthcare has no limits.
- Person of Contact: Emmy Elguizaoui
- Designation: Communications Director
- Details
- Written by: Atia Vision
- Category: Press Releases
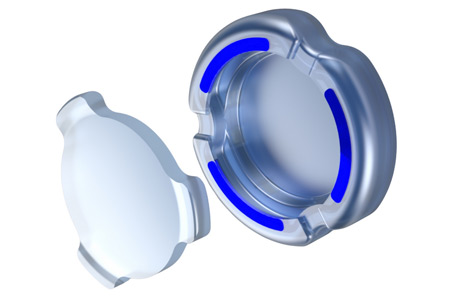
Atia Vision, Inc., a Shifamed portfolio company, announced it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin a traditional feasibility clinical study of its OmniVu™ Lens System. This novel intraocular lens is designed to restore dynamic range of vision following cataract surgery, going beyond the capabilities of current presbyopia-correcting lenses.
- Company Name: Atia Vision
- Company/Org Logo:

- About Company: Atia Vision is developing a modular presbyopia-correcting intraocular lens to address the full range of vision for patients with cataracts and presbyopia. The accommodating lens technology is designed to provide superior visual outcomes with an excellent safety profile and be uniquely customizable to address patients' evolving vision needs. Founded in 2014, Atia Vision's technology originated from the Shifamed portfolio, a Silicon Valley-based medical innovation hub.
- Person of Contact: Silvana Guerci-Lena
- Details
- Written by: Stryker
- Category: Press Releases
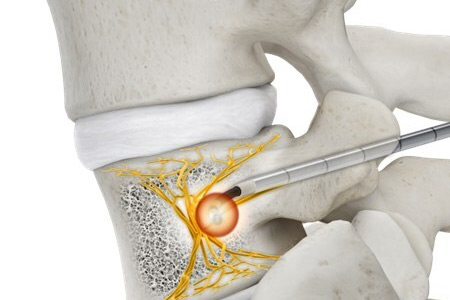
Stryker a global leader in medical technologies, announced that its OptaBlate basivertebral nerve ablation system (OptaBlate BVN) received 510(k) clearance from the U.S. Food and Drug Administration. OptaBlate BVNA is used in a targeted minimally invasive procedure providing long-lasting† vertebrogenic pain relief1.
- Company Name: Stryker
- Company/Org Logo:

- About Company: Stryker is a global leader in medical technologies and, together with its customers, we are driven to make healthcare better. We offer innovative products and services in MedSurg, Neurotechnology and Orthopaedics that help improve patient and healthcare outcomes. Alongside our customers around the world, we impact more than 150 million patients annually.
- Person of Contact: Beth Sizemore
- Designation: Senior Director, Communications
- Details
- Written by: Boston Scientific Corporation
- Category: Press Releases
Boston Scientific Corporation announced positive 12-month primary endpoint results from the second phase of the ADVANTAGE AF clinical trial evaluating the use of the FARAPULSE™ Pulsed Field Ablation (PFA) System* and adjunctive use of the FARAPOINT™ PFA Catheter in patients with persistent atrial fibrillation (AF). Key findings from the study were presented at the second annual PFA Live Case Summit in San Diego and simultaneously published in Circulation.
- Company Name: Boston Scientific Corporation
- Company/Org Logo:

- About Company: Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 45 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of health care. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions.
- Person of Contact: Becca Johnson
- Details
- Written by: SonoMotion, Inc.
- Category: Press Releases
SonoMotion, a medical device company developing noninvasive solutions for kidney stones, announced it has been awarded a $2.2 million NIH grant and successfully completed patient enrollment in the SOUND Break Wave pivotal clinical trial.
- Company Name: SonoMotion, Inc.
- Company/Org Logo:

- About Company: SonoMotion, a venture capital backed startup based in San Mateo, CA, is a clinical stage medical technology company developing next-generation, noninvasive solutions for the treatment of kidney stones. The SonoMotion Break Wave device is an investigational device that is not approved for sale in the United States or elsewhere.
- Details
- Written by: Medline Industries, LP
- Category: Press Releases
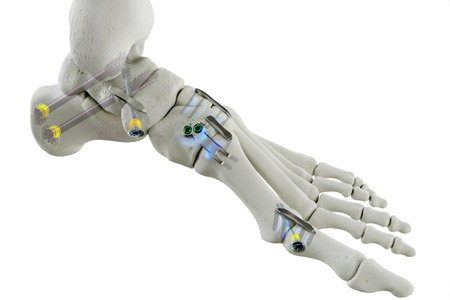
Medline UNITE, a leader in the foot and ankle surgery market, announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its latest implant family, the REFLEX® HYBRID Nitinol Implant System.
- Company Name: Medline Industries, LP
- Company/Org Logo:

- About Company: Medline is the largest provider of medical-surgical products and supply chain solutions serving all points of care. Through its broad product portfolio, resilient supply chain and leading clinical solutions, Medline helps healthcare providers improve their clinical, financial and operational outcomes. Headquartered in Northfield, Illinois, the company employs more than 43,000 people worldwide and operates in more than 100 countries and territories.
- Details
- Written by: SWS Medical
- Category: Press Releases
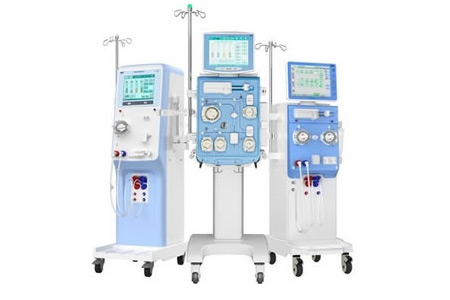
SWS Medical, a leading blood purification solution provider in China. For over two decades, SWS has cemented itself as a key player in dialysis, exporting to over 80 countries and securing 200 patents. It leads domestic dialysis machine brands in China with a 15.9% market share in 2024, rising to 27.11% in Feb 2025.
- Company Name: SWS Medical
- Company/Org Logo:

- About Company: Founded in 2001, SWS Hemodialysis Care Co., Ltd. (“SWS”) has been committed to the field of blood purification for over two decades. We are committed to prioritizing patient welfare and ensuring the delivery of safe and effective products and services as part of our ongoing responsibility and mission. Through technological advancements and ongoing enhancements, it has established itself as the sole company in China's blood purification sector to possess a comprehensive dialysis industrial chain.

- Details
- Written by: 4WEB Medical
- Category: Press Releases
4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary TRUSS Implant Technology™, announced that the company has surpassed the 100,000 spine procedure milestone, showing widespread adoption due to the technology's unique benefits. 4WEB has the distinction of being the first company to achieve 510(k) clearance for a spine implant manufactured with 3D printing technology.
- Company Name: 4WEB Medical
- Company/Org Logo:

- About Company: 4WEB Medical, founded in 2008 in Dallas, TX, is an orthopedic implant company. Thirty years of research in topological dimension theory led to the discovery of a novel geometry, the 4WEB, that can be used as a building block to create high-strength, lightweight web structures. The company leveraged this breakthrough to develop 4WEB Medical's proprietary TRUSS implant platform which was the first 510(k) cleared implant manufactured with 3D printing technology. The 4WEB Medical product portfolio includes the Cervical Spine TRUSS System™, Stand Alone Cervical Spine TRUSS System™, Anterior Spine TRUSS System™, Stand Alone Anterior Spine TRUSS System™, Posterior Spine TRUSS System™ and the Lateral Spine TRUSS System™.
- Exactech Secures a Groundbreaking Patent, Paving the Way for Next-Generation Personalized Surgical Planning
- Avatar Medical Vision Receives CE Marking
- Moon Surgical to Accelerate AI Capabilities in Maestro System with NVIDIA Isaac for Healthcare
- Silicon Labs unveils BG29, the future of Bluetooth® LE in miniature devices
- GT Metabolic™ expands the MagDI™ System with the FDA clearance of the 50mm magnet
