Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: BD
- Category: Press Releases
BD a leading global medical technology company, announced the commercial release of the BD Neopak™ XtraFlow™ Glass Prefillable Syringe and the latest capacity expansion of the BD Neopak™ Glass Prefillable Syringe platform to serve the growing market for biologic therapies.
- Company Name: BD
- Company/Org Logo:

- About Company: BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers,
- Person of Contact: Fallon McLoughlin
- Details
- Written by: Epitomee Medical Ltd.
- Category: Press Releases
Epitomee Medical Ltd. announced that the United States Food and Drug Administration (FDA) has officially cleared the Epitomee® Capsule, a cutting-edge ingestible medical device designed to support weight management in adults with a Body Mass Index (BMI) of 25–40 kg/m² alongside diet and exercise. This novel, drug-free treatment provides a new option for millions of health-conscious individuals in the U.S.
- Company Name: Epitomee Medical Ltd
- Company/Org Logo:

- About Company: Epitomee Medical Ltd, a public company (TASE: EPIT), co-founded in 2005 by Shimon Eckhouse, PhD and led by CEO Dan Hashimshony PhD. Shimon co-founded Syneron Medical Ltd. (Nasdaq: ELOS), Lumenis (formally ESC Medical) and co-founded Ventor Medical Technologies, which was acquired by Medtronic. Dan was the founding CEO of Dune Medical Devices, a surgical oncology company (acquired by Dilon Technologies). Prior to that, Dan was with X-Technologies (acquired by Guidant in 2003) and Sela Semiconductor (acquired by Camtek). The company is a pioneering health solutions company, committed to advancing innovative therapies. With focus on safety, efficacy, and improving quality of life, Epitomee Medical strives to be at the forefront of transformative healthcare solutions. The company is advancing two major fields: weight management and biologic drug delivery. In addition to its flagship weight management solution.
- Person of Contact: Alon Heth
- Details
- Written by: SamanTree Medical
- Category: Press Releases
SamanTree Medical, a leader in innovative surgical imaging solutions announced that it has received FDA 510(k) clearance of its Histolog® Scanner for imaging of the internal microstructure of tissues including, but not limited to, the identification of cells, vessels and their organization or architecture. This groundbreaking Swiss made device is designed to provide real-time, highresolution imaging of fresh tissue surfaces, offering surgeons and pathologists this information for excised tissue.
- Company Name: SamanTree Medical
- Company/Org Logo:

- About Company: SamanTree Medical is committed to enhancing clinical outcomes through the development of advanced imaging technologies. The Histolog® Scanner, now FDA-cleared, offers realtime, high-resolution imaging of excised tissue surfaces, providing physicians with this information for immediate assessments. Following the initial commercialization in Europe, the Histolog® Scanner is poised to make a significant impact in the U.S. healthcare market.
- Person of Contact: Olivier Delporte

- Details
- Written by: Hyperfine, Inc.
- Category: Press Releases
Hyperfine, Inc. the groundbreaking health technology company that has redefined brain imaging with the first FDA-cleared portable magnetic resonance (MR) brain imaging system—the Swoop® system—announced a collaboration with the Medical University of South Carolina to assess the brains of astronauts before and after embarking on the Polaris Dawn mission.
- Company Name: Hyperfine, Inc.
- Company/Org Logo:

- About Company: Hyperfine, Inc. (Nasdaq: HYPR) is the groundbreaking health technology company that has redefined brain imaging with the Swoop® system—the first FDA-cleared, portable, ultra-low-field, magnetic resonance brain imaging system capable of providing imaging at multiple points of care. The mission of Hyperfine, Inc. is to revolutionize patient care globally through transformational, accessible, clinically relevant diagnostic imaging. Founded by Dr. Jonathan Rothberg in a technology-based incubator called 4Catalyzer, Hyperfine, Inc. scientists, engineers, and physicists developed the Swoop® system out of a passion for redefining brain imaging methodology and how clinicians can apply accessible diagnostic imaging to patient care.
- Person of Contact: Dana Schroeder

- Details
- Written by: Thermo Fisher
- Category: Press Releases
Thermo Fisher Scientific, the world leader serving science, announced the latest recipients of the Oncomine Clinical Research Grant, designed to support emerging research on molecular profiling in oncology and to help democratize the future of precision medicine.
- Company Name: Thermo Fisher
- Company/Org Logo:

- About Company: Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue over $40 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating life sciences research, solving complex analytical challenges, increasing productivity in their laboratories, improving patient health through diagnostics or the development and manufacture of life-changing therapies, we are here to support them. Our global team delivers an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, Patheon and PPD.

- Details
- Written by: ThinkLite
- Category: Press Releases
ThinkLite Air, a pioneer in indoor air quality (IAQ) solutions, proudly announces its collaboration with Medxcel, a subsidiary of Ascension and most recently, Prime Healthcare, to enhance patient safety at a Texas children's hospital.
- Company Name: ThinkLite
- Company/Org Logo:

- About Company: ThinkLite Air is an industry leader in IAQ monitoring and purification solutions. Leveraging proprietary AI technology to autonomously detect and remove harmful airborne pollutants in real-time, ThinkLite Air designs and manufactures innovative products that improve health, safety, and productivity. With a commitment to improving air quality backed by extensive research, ThinkLite Air is a trusted partner for organizations dedicated to health and energy efficiency.

- Details
- Written by: StatLab Medical
- Category: Press Releases
StatLab Medical Products a leading global developer and manufacturer of medical diagnostic supplies and equipment, announced that it has reached an agreement to acquire Diapath S.p.A. (“Diapath”), a prominent Italian manufacturer of histology and cytology products and equipment.
- Company Name: StatLab Medical
- Company/Org Logo:

- About Company: StatLab Medical Products has been dedicated to helping anatomic pathology laboratories provide the best possible patient care since 1976. We offer an extensive portfolio of self-manufactured consumables and labeling and tracking equipment from eight manufacturing sites in the United States, United Kingdom, and Germany. Our global operational footprint powered by over 600 mission-driven colleagues delivers a dependable and resilient supply chain of high-quality products and solutions, and a customer-centric approach inspires us to deliver reliability, innovation, and quality in every interaction.
- Details
- Written by: AISAP
- Category: Press Releases
AISAP, a medical technology company developing AI-powered Point-of-Care Assisted Diagnosis (POCAD™) solutions to transform medical ultrasound, has announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its first-of-a-kind, AI-powered AISAP CARDIO point-of-care ultrasound (POCUS) software platform.
- Company Name: AISAP
- Company/Org Logo:

- About Company: AISAP LTD is a pioneering company at the intersection of artificial intelligence and healthcare with a mission to transform healthcare delivery through its flagship product, AISAP CARDIO, which for the first time delivers POCAD™ (Point-of-Care Assisted Diagnosis). The platform enables clinicians to perform comprehensive, AI-powered ultrasound diagnostics anywhere, anytime, ensuring that advanced medical care is accessible at the patient’s bedside, whether in a hospital, clinic or rural setting.
- Person of Contact: Sam Choinski

- Details
- Written by: Alafia Ai, Inc.
- Category: Press Releases
Alafia Ai, Inc. announces the general availability of AIVAS, an all-in-one interactive high-performance personal supercomputer designed to revolutionize the development and deployment of critical artificial intelligence (AI) software applications in healthcare.
- Company Name: Alafia Ai, Inc.
- Company/Org Logo:

- About Company: Alafia Ai, Inc. provides a high-performance precision medicine platform designed to accelerate scientific and medical discoveries across hospitals, cancer and imaging centers, research institutions, pharmaceutical organizations, and biotechnology companies. Rooted in the belief that personalized care is fundamental to healthcare excellence, ALAFIA’s vision is to create an intelligent and adaptable healthcare ecosystem ensuring that every patient receives the highest standard of care.
- Person of Contact: Camilo Buscaron
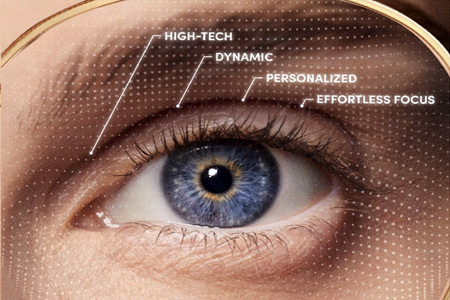
- Details
- Written by: LensCrafters
- Category: Press Releases
LensCrafters, part of EssilorLuxottica and one of the largest optical retail brands in North America, announced the launch of LensCrafters Adaptive™ Progressive Lenses, the retailer's most advanced, tailored and highly personalized progressive lenses delivering an optimal visual experience.
- Company Name: LensCrafters
- Company/Org Logo:

- About Company: LensCrafters, the leading optical retailer in North America, was founded in 1983 and currently operates over 1,000 stores in the U.S., Canada and Puerto Rico. With a mission of helping people look and see their best, LensCrafters has a passion for vision care and offers the best selection of the latest trends in eyewear from leading designer brands as well as incomparable personalized service from Doctors of Optometry located at or next to its stores. LensCrafters opened its first Macy's location in April 2016 and currently has five flagship stores in New York City, San Francisco, Palo Alto and Toronto, the first flagship in Canada that opened in July 2023. The brand's trusted doctors and associates continue to make an impact by giving the gift of vision through the company's partner efforts with OneSight EssilorLuxottica Foundation, providing access to quality vision care and glasses in underserved communities worldwide. LensCrafters is currently the number one contributor to OneSigh
- THINK Surgical Receives FDA 510(k) Clearance for TMINI Miniature Robotic System
- ICU Stays and Better Patient Outcomes with Ceribell Point-of-Care EEG Compared to Conventional EEG
- Vectorious Medical Technologies Announces First U.S. Patient Implanted with the V-LAP Left Atrial Pressure Sensor
- VisionAir Solutions Celebrates Milestone and New Collaboration
- NextStep Arthropedix to Launch TheRay™ Hip System
