Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',
- Details
- Written by: Clear Guide Medical
- Category: Press Releases
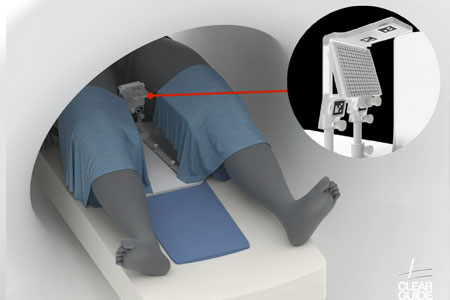
Clear Guide Medical proudly announces the FDA clearance for the CLEAR GUIDE SCENERGY computer aided instrument guidance system, alongside the new TP Access Tool with SteriGRID™.
- Company Name: Clear Guide Medical
- Company/Org Logo:

- About Company: Clear Guide Medical, Inc., a privately-held company headquartered in Baltimore, MD, develops innovative medical devices utilizing AI and AR with Computer-Assisted Instrument Guidance for minimally invasive medical procedures.
- Person of Contact: Logan Garrett
- Details
- Written by: Aerin Medical Inc
- Category: Press Releases
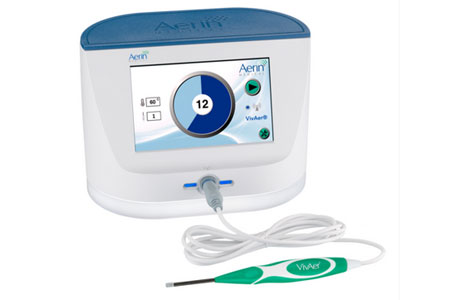
Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, announced that Rhinology Journal has published positive two-year outcomes from the VATRAC trial.
- Company Name: Aerin Medical Inc
- Company/Org Logo:

- About Company: Aerin Medical is a privately held, venture-backed company, with U.S. offices in California and Texas. Aerin’s mission is to expand access to meaningful relief for millions of patients suffering from chronic ENT conditions. The company’s products, VivAer® for nasal airway obstruction and RhinAer® for chronic rhinitis, leverage Aerin’s proprietary temperature-controlled technology, which allows ENT physicians to reliably improve patients’ symptoms with unique technologies that are appealing alternatives to invasive surgery. More than 100,000 patients have been treated with Aerin Medical products to date.
- Person of Contact: Laura Morgan
- Designation: Manager
- Details
- Written by: ViiV Healthcare
- Category: Press Releases
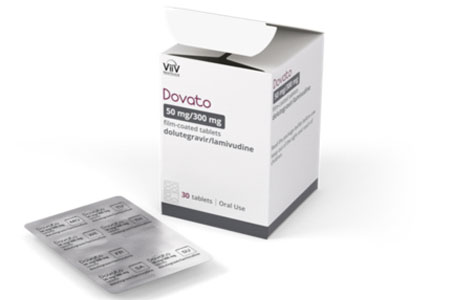
ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, announced Dovato (dolutegravir/ lamivudine) is now available in a blister pack in the U.S.
- Company Name: ViiV Healthcare
- Company/Org Logo:

- About Company: ViiV Healthcare is a global specialist HIV company established in November 2009 by GSK (LSE: GSK) and Pfizer (NYSE: PFE) dedicated to delivering advances in treatment and care for people living with HIV and for people who are at risk of acquiring HIV. Shionogi became a ViiV shareholder in October 2012. The company’s aims are to take a deeper and broader interest in HIV and AIDS than any company has done before and take a new approach to deliver effective and innovative medicines for HIV treatment and prevention, as well as support communities affected by HIV.
- Person of Contact: Rachel Jaikaran
- Details
- Written by: Edwards
- Category: Press Releases
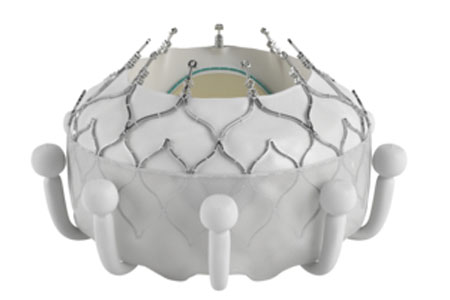
Edwards Lifesciences Corporation announced the company’s EVOQUE tricuspid valve replacement system is the first transcatheter therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation (TR).
- Company Name: Edwards
- Company/Org Logo:

- About Company: Edwards Lifesciences is the global leader of patient-focused innovations for structural heart disease and critical care monitoring. We are driven by a passion for patients, dedicated to improving and enhancing lives through partnerships with clinicians and stakeholders across the global healthcare landscape. This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, but are not limited to, statements made by Mr. Chopra and Dr. Kodali, and statements regarding expected product benefits, patient outcomes, objectives and expectations and other statements that are not historical facts. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict. Our forward-looking statements
- Person of Contact: Loree Bowen

- Details
- Written by: PrepMD
- Category: Press Releases
PrepMD, the leading cardiac healthcare solutions provider, announced the accreditation of their online healthcare training programs for Nursing Continuing Education Units (CEUs).
- Company Name: PrepMD
- Company/Org Logo:

- About Company: PrepMD is the premier tech-enabled solutions company empowering healthcare providers to achieve exceptional standards of cardiac care. With 15+ years of industry expertise, the company offers a comprehensive portfolio of device clinic solutions, featuring exclusive in-clinic staffing services and best-in-class remote monitoring offerings. The company continues to be a leading provider of customized training solutions to device clinics, corporations, and individuals globally. PrepMD is dedicated to optimizing patient outcomes and driving innovation in cardiac care.

- Details
- Written by: Accuray Incorporated
- Category: Press Releases
Accuray Incorporated announced that the Providence Swedish Radiosurgery Center in Seattle, Washington is enhancing its cancer treatment capabilities with the purchase of the latest generation CyberKnife® S7™ System, the hospital's second CyberKnife radiation delivery device purchase.
- Company Name: Accuray Incorporated
- Company/Org Logo:

- About Company: Accuray is committed to expanding the powerful potential of radiation therapy to improve as many lives as possible. We invent unique, market-changing solutions that are designed to deliver radiation treatments for even the most complex cases—while making commonly treatable cases even easier—to meet the full spectrum of patient needs. We are dedicated to continuous innovation in radiation therapy for oncology, neuro-radiosurgery, and beyond, as we partner with clinicians and administrators, empowering them to help patients get back to their lives, faster. Accuray is headquartered in Madison, Wisconsin, with facilities worldwide.
- Person of Contact: Beth Kaplan
- Designation: Public Relations Director
- Details
- Written by: Olympus Corporation of the Americas
- Category: Press Releases
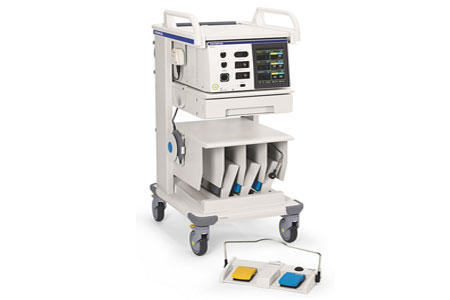
Olympus Corporation, a global medical technology company committed to making people's lives healthier, safer and more fulfilling, announced full market availability of its new ESG-410™ Surgical Energy Platform for conventional monopolar and bipolar, advanced energy applications now including ultrasonic dissection and hybrid energy.
- Company Name: Olympus Corporation of the Americas
- Company/Org Logo:

- About Company: A leading medical technology company, Olympus uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Olympus' portfolio includes endoscopes, laparoscopes, and video imaging systems, as well as surgical energy devices, system integration solutions, medical services, and a wide range of endotherapy instruments.
- Details
- Written by: IlluminOss Medical
- Category: Press Releases
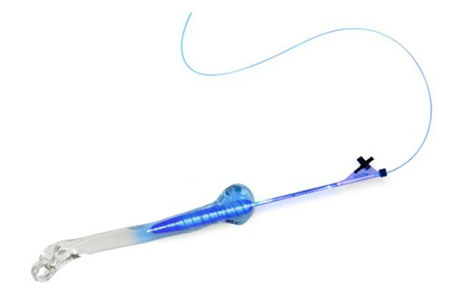
IlluminOss Medical announces it has reached the important benchmark of having placed 10,000 of its patient conforming implants designed for the treatment of geriatric fractures.
- Company Name: IlluminOss Medical
- Company/Org Logo:

- About Company: Headquartered in East Providence, RI, IlluminOss is a privately held, commercial-stage medical device company offering a unique, minimally invasive technology for fracture repair and stabilization. The company utilizes a light-curable monomer contained within an expandable balloon to create a patient-conforming intramedullary implant for bone stabilization. The revolutionary, minimally invasive technology is applicable for repair and treatment of osteoporotic and compromised bone. The IlluminOss system is CE-marked and FDA-cleared for a variety of anatomical sites, with further indications pending.
- Person of Contact: Lisa Holt

- Details
- Written by: BD
- Category: Press Releases
BD a leading global medical technology company, announced a collaboration agreement with Hamilton, a leading global manufacturer of laboratory automation technology, to develop automated applications together with robotics-compatible reagent kits to enable greater standardization and reduced human error when conducting large-scale single-cell multiomics experiments.
- Company Name: BD
- Company/Org Logo:

- About Company: BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers,
- Person of Contact: Troy Kirkpatrick
- Designation: Public Relations
- Details
- Written by: Single Pass, Inc
- Category: Press Releases
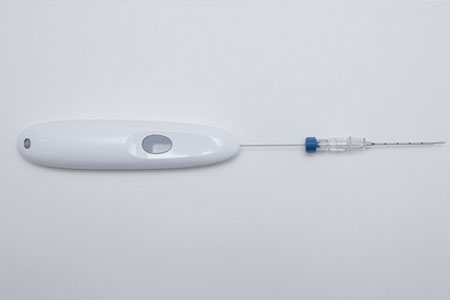
Single Pass, a leading provider of innovative medical devices, is pleased to announce an exclusive distribution agreement with Mermaid Medical Group, a renowned global medical technology company.
- Company Name: Single Pass, Inc
- Company/Org Logo:

- About Company: Single Pass is a leading provider of innovative medical devices, dedicated to improving patient care and procedural outcomes. With a focus on developing advanced solutions for biopsy closure procedures, Single Pass aims to revolutionize the field of interventional medicine.
- Person of Contact: Bill Colone
- Restor3d scales personalized portfolio post Conformis acquisition
- Pursuant Health reveals the first FDA-cleared Retinal Imaging Kiosk in on-demand healthcare
- Achieving a significant milestone, BizLink Robotic Solutions France celebrates the delivery of its 100th ORION patient positioning system
- LASEROPTEK Co. Ltd. collaborates with The Pinnacle Health Group to boost CPT coding support for U.S. customers using the FDA 510(k) cleared PALLAS & PALLAS Premium UVB Laser Systems
- BizLink once again proves its ability to innovate in healthcare robotics
