Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',

- Details
- Written by: EyeCheq
- Category: Press Releases
EyeCheq, a leading innovator in eye care technology with its fully autonomous eye screening platform, is pleased to announce the expansion of their Leadership Team. Dr. Ruwan Silva will serve as Chief Medical Officer and Christopher Grant as EVP of Operations to strengthen EyeCheq's scientific and operational expertise.
- Company Name: EyeCheq
- Company/Org Logo:

- About Company: EyeCheq, the world's first fully automated eye health platform, is revolutionizing eye care. The company's mission is to provide accessible, accurate, and convenient eye health solutions to individuals worldwide. EyeCheq's innovative products and services aim to improve early detection of eye conditions, ultimately enhancing the quality of life for patients. With a strong focus on patient-centric solutions, EyeCheq aims to empower primary care professionals, health plans, and retail health care settings to provide patients with the highest standard of care.

- Details
- Written by: Genezen
- Category: Press Releases
Genezen, a leading cell and gene therapy Contract Development and Manufacturing Organization (CDMO),announced the closing of an $18.5 million follow-on growth equity investment led by Ampersand Capital Partners.
- Company Name: Genezen
- Company/Org Logo:

- About Company: Genezen is a contract development and manufacturing organization (CDMO) with a decade's experience at the heart of the rapid growth in the gene and cell therapy market. Genezen is a leader in the supply of retroviral vectors, lentiviral vectors, and AAV. Led by an extremely experienced team, a science-first approach influences continual investment in scalable, high-yield manufacturing processes and best-in-class technologies.

- Details
- Written by: Xēnix Medical
- Category: Press Releases
Xēnix Medical, a surgical implant company focused on the development of novel science-based solutions for patients requiring spinal fusion surgery, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the SOLACE™️ Sacroiliac Fixation System with proprietary NANOACTIV™️ surface technology and compatibility with StealthStation® Navigation.
- Company Name: Xēnix Medical
- Company/Org Logo:

- About Company: Xēnix Medical is a surgical implant company focused on the development of novel solutions to advance the science of fusion engineering to enhance the treatment of various pathologies for patients requiring spinal fusion surgery. The company, headquartered in Orlando, Florida, is in active development of multiple unique implant technology platforms and markets its line of spinal implant devices in the United States through a network of independent distributors.
- Person of Contact: Ryan Phillips
- Designation: President
- Details
- Written by: Lazurite
- Category: Press Releases
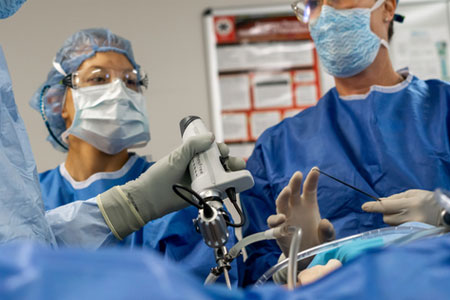
Medical device and technology company Lazurite® announced that its ArthroFree® Wireless Camera System will be available at the Arthroscopy Association of North America (AANA) Advanced Knee and Shoulder course on Oct. 20-22, and the Innovations in Hip Arthroscopy lab course on Nov. 3-4, at the AANA Orthopaedic Learning Center in Rosemont, Illinois.
- Company Name: Lazurite
- Company/Org Logo:

- About Company: Lazurite designs medtech devices. Its ArthroFree® System is the first wireless camera with FDA clearance for arthroscopy and general endoscopy. The ArthroFree System is designed to enhance the surgeon's focus during the critical moments of surgery by enabling untethered movement and dexterity. It promises to increase the safety and efficiency of minimally invasive surgical procedures while decreasing the cost. Lazurite’s IP portfolio includes the high-efficiency Meridiem® light technology, wireless communication technology, and products in development. Lazurite’s three-year vision (by 2026): Modernize 1,000 operating rooms for the wireless age.
- Person of Contact: Patrick Gallagher
- Details
- Written by: Wind River
- Category: Press Releases
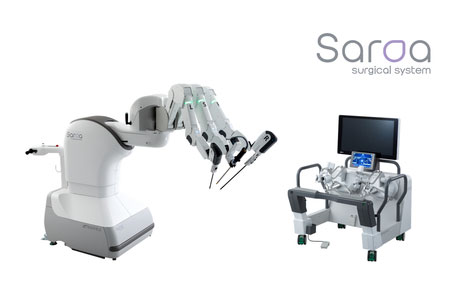
-Wind River®, a global leader in delivering software for mission-critical intelligent systems, announced that RIVERFIELD Inc., is using VxWorks® to develop the Saroa Surgical System, a surgical assist robot.
- Company Name: Wind River
- Company/Org Logo:

- About Company: Wind River is a global leader in delivering software for mission-critical intelligent systems. For more than four decades, the company has been an innovator and pioneer, powering billions of devices and systems that require the highest levels of security, safety, and reliability. Wind River software and expertise are accelerating digital transformation across industries, including automotive, aerospace, defense, industrial, medical, and telecommunications. The company offers a comprehensive portfolio supported by world-class global professional services and support and a broad partner ecosystem.
- Person of Contact: Jenny Suh
- Details
- Written by: Linear Health Sciences
- Category: Press Releases
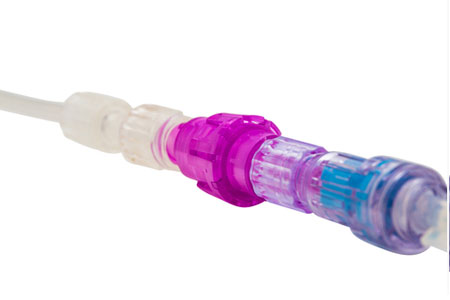
Medical device company Linear Health Sciences announced it received an expanded indication from the U.S. Food & Drug Administration (FDA) for its Orchid SRV™ safety release valve. The expanded indication means that the Orchid SRV may now be used with all IV access methods.
- Company Name: Linear Health Sciences
- Company/Org Logo:

- About Company: Linear Health Sciences is a medical device company that has developed a proprietary, breakaway safety valve technology designed to improve the use of medical tubing in hospitals. The platform technology was developed to increase the safety and satisfaction of patients, caregivers, and healthcare facilities, while dramatically reducing costs. The company’s initial products include the Orchid SRV for use in IV catheter therapy and the Orchid SRV Type D device for use in surgical/wound, nephrostomy and abscess drainage.
- Details
- Written by: American Regent Inc
- Category: Press Releases
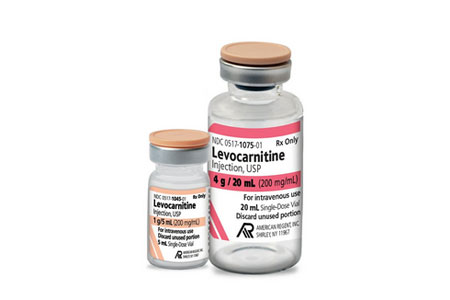
American Regent announces the release and availability of Levocarnitine Injection, USP, FDA-approved, "AP" rated, and therapeutically equivalent to Carnitor®. Levocarnitine is indicated for the acute and chronic treatment of patients with an inborn error of metabolism which results in secondary carnitine deficiency, and for the prevention and treatment of carnitine deficiency in patients with end-stage renal disease who are undergoing dialysis.
- Company Name: American Regent Inc
- Company/Org Logo:

- About Company: American Regent, Inc.®, a Daiichi Sankyo Group company, is an industry-leading injectable manufacturer. For over 50 years, American Regent has been developing, manufacturing, and supplying quality generic and branded injectables for healthcare providers. For more than 20 years, we have been a leader in IV iron therapy. American Regent is committed to US-based manufacturing. To that end, over the last several years we have made significant investments in expanding and modernizing our manufacturing facilities in Ohio and New York. This expansion will nearly double our capacity and allow us to better serve our customers now and in the future. Speed counts. Flexibility matters. Reliability and quality are paramount. Because patients should never have to wait for the medications they need.

- Details
- Written by: ClariMed
- Category: Press Releases
ClariMed, Inc., the first end-to-end MedTech services partner to prioritize usability as the core of their integrated, human-centric approach to medical product development, announced the appointment of Sandrine Parkins as Head of Operations for Harvey Medical, a ClariMed company.
- Company Name: ClariMed
- Company/Org Logo:

- About Company: ClariMed is a human-centered development and regulatory practice for medical products developed by Pharmaceutical and Medical device manufacturers. Our best-of-breed professional services cultivate innovation while ensuring the safe and effective use of medical products.
- Person of Contact: Monique Garrett
- Designation: Manager
- Details
- Written by: Quanta Dialysis Technologies
- Category: Press Releases
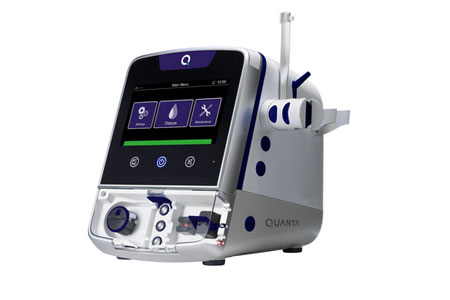
Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, announced that it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for indication expansion of the Quanta™ Dialysis System, a compact and easy-to-use hemodialysis device.
- Company Name: Quanta Dialysis Technologies
- Company/Org Logo:

- About Company: Quanta Dialysis Technologies is committed to making dialysis accessible to every patient in every setting with its Quanta Dialysis System. As a portable device with performance comparable to larger, traditional machines, the Quanta Dialysis System is a modular and powerful solution that provides the clinical versatility needed to deliver dialysis care across multiple settings. With a simple-to-use and intuitive user interface, it is designed to be operated by a broad range of users to bring dialysis directly to patients. The Quanta Dialysis System is commercially available in the United Kingdom for home and hospital use. In the United States, it is FDA-cleared (K222067) for use in chronic and acute care settings, with treatment types including intermittent hemodialysis (IHD), sustained low efficiency dialysis (SLED), prolonged intermittent renal replacement therapy (PIRRT), slow continuous ultrafiltration (SCUF), and continuous venovenous hemodialysis (CVVHD).
- Person of Contact: Melinda Freson
- Designation: CEO
- Details
- Written by: GT Molecular
- Category: Press Releases
GT Molecular, Inc., a company providing ultrasensitive PCR assays for wastewater-based epidemiology testing, pathogen detection, and cancer research is pleased to announce an agreement with Roche to provide a highly multiplexed respiratory panel for wastewater surveillance on the new Roche Digital LightCycler® System.
- Company Name: GT Molecular
- Company/Org Logo:

- About Company: With its GT-Plex™ patented assay technology, GT Molecular is a leader in providing ultrasensitive, multiplexed digital PCR and qPCR tests for cancer research and wastewater-based epidemiology. GTM's technology provides a user friendly, out-of-box solution for rapid deployment with reliable measurements to detect as little as 3-4 molecules of target nucleic acid.
- New Study Finds That Masimo SedLine® Patient State Index
- HemoSonics Receives Vizient Innovative Technology Contract for Quantra Hemostasis System
- Quanta™ Receives FDA 510(k) Clearance for Expanded Indication of Continuous Renal Replacement Therapies
- XRP Healthcare and Spiritus Medical Partner to Revolutionize Healthcare in Africa
- PacBio Begins Commercialization of the Onso Short-Read Sequencing System
