Healthcare Press Releases
— To learn more on how to reach a suitable target audience by distributing / publicizing your content(the latest happenings about your company) as in 'Press Releases' through 'Global Healthcare Technology',

- Details
- Written by: Codesteri
- Category: Press Releases
CodeSteri, a leading provider of disinfection solutions, is proud to announce the launch of PlaClin-M, a revolutionary device designed to help ensure a clean and safe environment in offices, commercial establishments, and public spaces. CodeSteri started from the Korean government project to combat new infectious diseases by the incumbent professor and MD of Hanyang University Emergency Medicine, Seoul.
- Company Name: Codesteri
- Company/Org Logo:

- About Company: CODESTERI started in 2015 as a research project funded by the Korea Ministry of Science and ICT to develop disinfection systems, and the company was established in 2018 as a laboratory start-up company at Hanyang University in Seoul as a special purpose company to distribute the developed disinfection technology and equipment.
- Details
- Written by: Ancora Heart, Inc.
- Category: Press Releases
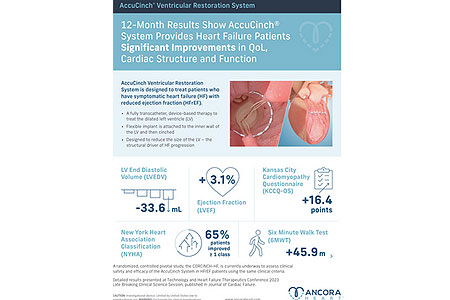
Ancora Heart, Inc., a company developing a completely transcatheter device-based therapy to address heart failure (HF), announced that patients treated with the investigational AccuCinch® Ventricular Restoration System demonstrated improvement in HF patient outcomes and beneficial changes in the structure of the heart. The 12-month data were presented as part of a late-breaking clinical science session at the Technology and Heart Failure Therapeutics conference (THT 2023) and simultaneously published in the Journal of Cardiac Failure.
- Company Name: Ancora Heart, Inc.
- Company/Org Logo:

- About Company: Ancora Heart is a medical device company dedicated to providing new treatment options for people with heart failure (HF). The company’s proprietary AccuCinch® Ventricular Restoration System is the only completely transcatheter device designed to restore the structure and function of the enlarged left ventricle of the heart, thereby addressing the fundamental issue in the progression of heart failure in patients with reduced ejection fraction (HFrEF).. Ancora Heart is a privately held company located in Santa Clara, Calif.
- Person of Contact: Jessica Volchok
- Designation: PR Manager

- Details
- Written by: TransMed7, LLC
- Category: Press Releases
TransMed7, LLC announced the first clinical use of VacuPac®, a new self-contained external vacuum-assist attachment with a SpeedBird Universal and a Concorde US from the SpeedBird and Concorde families of vacuum-assisted, Single Insertion – Multiple Collection (SIMC® ) breast biopsy devices.
- Company Name: TransMed7, LLC
- Company/Org Logo:

- About Company: TransMed7, LLC is a medical and technology based organization focused on the highly efficient development of innovative, minimally invasive medical devices aimed at providing new solutions for doctors and their patients. With particular expertise in oncologic, regenerative, and cardiovascular disease, TransMed7 and its team of clinicians, scientists, and engineers have developed a portfolio of next-generation platform devices that are expected to be market leaders in their targeted fields of medicine. TransMed7 accomplishes this through application of a wholly new approach in its business plan and structure, enabling these new transformational technologies rapid development through commercial manufacturing or where appropriate, handoff through acquisition to our strategic partners.
- Person of Contact: Jonathan J. Coyne
- Designation: Vice President
- Details
- Written by: Lumicell, Inc.
- Category: Press Releases
Lumicell, Inc., a privately held company focused on innovative fluorescence-guided imaging technologies for cancer surgery, announced a New Drug Application (NDA) for its LUMISIGHT™ Optical Imaging Agent has been submitted to the U.S. Food and Drug Administration (FDA).
- Company Name: Lumicell, Inc.
- Company/Org Logo:

- About Company: Lumicell is a privately held company focused on improving surgical outcomes and reducing healthcare costs by utilizing its innovative fluorescence-guided surgical technologies to enable a more complete resection of cancer that may have otherwise been left behind. The company’s first product in development is the Lumicell Direct Visualization System, designed to illuminate cancerous tissue within the breast cavity during the initial lumpectomy procedure. Lumicell’s proprietary, pan-oncologic optical imaging agent LUMISIGHT is also being explored across a wide variety of solid tumor indications.
- Person of Contact: Joni Ramirez
- Designation: Communications Manager
- Phone: 323-532-0746

- Details
- Written by: Syft Technologies
- Category: Press Releases
Syft Technologies today announced the release of its next generation Selected Ion Flow Tube Mass Spectrometry (SIFT-MS) technology, Syft Tracer™, at Pittcon 2023. This innovation of real-time, direct injection mass spectrometry (MS) offers platform advancements such as greater sensitivity, unparalleled performance stability, and high reproducibility and repeatability that make the system an industry-scalable solution. Analytical workflows that require fast time to data, high throughput, and continuous operation will benefit significantly from Syft Tracer capabilities.
- Company Name: Syft Technologies
- Company/Org Logo:

- About Company: Syft was founded in 2002 and has over 150 professionals in 7 countries. Syft is considered the world leader in real-time, direct injection mass spectrometry with more than 20 years of SIFT-MS expertise. Syft instruments support a broad range of industries worldwide including semiconductor manufacturing, pharma and CDMOs, environmental protection, automotive, food, flavor and fragrance, and many more. We have offices throughout the world offering 24/7 service and support including those in New Zealand, Korea, Taiwan, Singapore, Germany and the U.S.
- Details
- Written by: SurgVision
- Category: Press Releases
SurgVision, a high-tech company developing pioneering solutions for fluorescence-guided surgical and interventional oncology, part of the Bracco Group, announces that it has received 510(k) clearance from the U.S. Food and Drug Administration for the EXPLORER AIR® II for use with Pafolacianine (CYTALUX, On Target Laboratories Inc.) during intraoperative fluorescence imaging.
- Company Name: SurgVision
- Company/Org Logo:

- About Company: SurgVision, part of Bracco Group, is a medical technology company with a mission to bring innovation to the imaging space. By leveraging imaging technologies, we are committed to enhancing clinical practice, improving outcomes, and reducing the cost of care.
- Person of Contact: Duccio Manetti
- Designation: Global Communications Director
- Phone: +39 340 9016191

- Details
- Written by: Bruker Corporation
- Category: Press Releases
Bruker announces that the United Kingdom is expanding its fundamental research infrastructure with recent orders for two 1.2 GHz Avance™ nuclear magnetic resonance (NMR) spectrometers for the University of Warwick and the University of Birmingham.
- Company Name: Bruker Corporation
- Company/Org Logo:

- About Company: Bruker is enabling scientists to make breakthrough discoveries and develop new applications that improve the quality of human life. Bruker’s high performance scientific instruments and high value analytical and diagnostic solutions enable scientists to explore life and materials at molecular, cellular and microscopic levels. In close cooperation with our customers, Bruker is enabling innovation, improved productivity and customer success in life science molecular and cell biology research, in applied and pharma applications, in microscopy and nanoanalysis, as well as in industrial applications. Bruker offers differentiated, high-value life science and diagnostics systems and solutions in preclinical imaging, clinical phenomics research, proteomics and multiomics, spatial and single-cell biology, functional structural and condensate biology, as well as in clinical microbiology and molecular diagnostics.
- Person of Contact: Justin Ward
- Designation: Sr. Director

- Details
- Written by: Prolira
- Category: Press Releases
Prolira BV, a high-tech company committed to enabling early and effective treatment of patients at risk of developing acute brain failure, announced it has received 510(k) clearance from the Food and Drug Administration (FDA) for the DeltaScan® Brain State Monitor to aid in the diagnosis of acute encephalopathy in hospitalized patients over 60 years of age.
- Company Name: Prolira
- Company/Org Logo:

- About Company: Prolira is a high-tech start-up that addresses a costly and growing healthcare problem: acute brain failure (acute encephalopathy and delirium), a serious response of the brain to common underlying medical conditions like post-operative infections, organ failures, and metabolic disorders. Every year, 20 million patients are at risk of acute brain failure in EU and US hospitals, which leads to long-term cognitive impairment, longer hospital stays, and increased healthcare costs. Prolira's DeltaScan® Brain State Monitor is the world's first EEG bedside device with a validated proprietary algorithm to support clinicians in their quest to optimize patient recovery.

- Details
- Written by: Picarro, Inc.
- Category: Press Releases
Picarro Inc., a leading provider of chemical metrology systems for advanced semiconductor fabs, announced the SLiM 100 Lithography Process Tool Monitoring System. The new 1-ppb class chemical metrology solution detects volatile organic compounds (VOCs) in the lithography process in real time, enabling semiconductor manufacturers to quickly take steps to prevent excursions and detect non-visual defects, thereby improving yield.
- Company Name: Picarro, Inc.
- Company/Org Logo:

- About Company: Picarro's Semiconductor business offers industry leading solutions for airborne molecular contamination (AMC) and chemical contaminant monitoring within process tools. Picarro's Semiconductor solutions respond to contaminants in seconds, not hours, enabling semiconductor manufacturers to quickly mitigate contamination events in cleanrooms, FOUP, and lithography process equipment – improving production yield.
- Person of Contact: Jake Thill
- Designation: Director

- Details
- Written by: Visby Medical
- Category: Press Releases
Visby Medical™ announced that it has received 510(k) clearance and was granted a CLIA waiver from the U.S. Food and Drug Administration for its second generation point of care (POC) test. The Visby Medical Sexual Health test is a fast, polymerase chain reaction (PCR) diagnostic test for the detection of sexually transmitted infections (STIs) caused by Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis in women.
- Company Name: Visby Medical
- Company/Org Logo:

- About Company: Visby Medical is transforming the order of diagnosis and treatment for infectious diseases so clinicians can test, talk with, and treat the patient in a single visit. The Company developed a proprietary technology platform that is the world's first instrument-free, single-use PCR platform that fits in the palm of your hand and rapidly tests for serious infections. The Visby Medical Sexual Health Test for women is the first step in a robust pipeline that is accelerating the delivery of fast and accurate, palm-sized PCR diagnostics to the point of care, and eventually for use at home.
- DISC Surgery Center to Provide Greater Access to Outpatient Spine Care
- PENTAX Medical INSPIRA™ Video Processor (EPK-i8020c)
- Nation's first pediatric hybrid intraoperative MRI neurosurgery suite opens at Children's Minnesota
- Co-Diagnostics, Inc. Initiates Clinical Evaluations for its At-Home and Point-of-Care Co-Dx PCR
- NovaSure® V5 Global Endometrial Ablation Device
